230 South 500 East, Suite 150
Salt Lake City, Utah 84102
*Dr. Layton is now at Eastern Virginia Medical School, Department of Otolaryngology
825 Fairfax Ave, Ste 510
Norfolk, VA 23507
Introduction
Vestibular function tests are performed on patients with various disorders of the peripheral vestibular system and the central nervous system. It is often times difficult to determine the exact site of lesion when considering vestibular signs, symptoms, or vestibular function test results. The most common diagnoses of the peripheral vestibular system include Benign Paroxysmal Positional Vertigo (BPPV), Meniere's disease, and Vestibular Neuritis (VN). There is significant literature available concerning Meniere's and BPPV;however, less is written concerning VN. Ruttin, in 1909, first described VN as "a sudden unilateral loss of vestibular function without auditory symptoms, occurring in an otherwise healthy person." According to Furman and Cass (2003), VN is a condition also known as labyrinthitis, vestibular neuronitis, and acute vestibulopathy of uncertain etiology. According to these authors, the terms are used interchangeably. In an article by Schuknecht and Kitamura entitled "Vestibular Neuritis" (1981), the terms vestibular neuritis, vestibular neuronitis and epidemic vertigo were used synonymously. They defined this as a disorder of the vestibular system without an associated auditory deficit or other disease of the central nervous system (CNS). For the purpose of this paper, we shall refer to this disorder at all times as VN. In our experience, acute-stage VN is characterized by extreme vertigo with associated nausea and vomiting, of which symptoms typically last from several hours to 2-3 days. Medical treatment is generally aimed at alleviating these symptoms via anti-dizzy and anti-nausea agents. Patients are rarely referred by the medical professional for vestibular function testing while still in the acute stage, though it is not uncommon for clinicians to see these patients once the acute symptoms have passed and they are left with residual symptoms of dizziness and dysequilibrium.
In 1967, Lachman & Stahle reported on 45 cases of acute VN over a six-year period. They reported eight essential features of VN, in order of importance, for the diagnosis to be made:
- vertigo, generally sudden and severe,
- spontaneous nystagmus, vigorous and beating away from the diseased labyrinth,
- unimpaired hearing [which differentiates VN from labyrinthitis],
- canal paresis, or in other words, reduced excitability, to caloric irrigation on the diseased side,
- prolonged course, from weeks to months,
- incidence highest in the third, fourth, and fifth decades of age,
- normal neurologic exam except for dysequilibrium and nystagmus, and
- recent history of infection.
- an acute unilateral peripheral vestibular disorder without an associated hearing loss [which differentiates VN from labyrinthitis],
- occurrence predominantly in middle age,
- a single episode of severe prolonged vertigo,
- decreased caloric response in the involved ear, and
- complete subsidence of the symptoms within six months.
On the fifth of the month, about a week after flu-like symptoms, this healthy, physically fit and active young man experienced sudden-onset vertigo and nausea, which was continuous and severe in nature. The next day he was seen by a local otolaryngologist, who, after ruling out possible stroke, referred him for a vestibular evaluation. One day later he underwent vestibular function testing (ENG and Posturography) at our center. At the time of the evaluation, he had had a three-day history of significant vertigo and nausea. He reported that day as being the worst symptomatically when compared to the previous two days and described his symptoms as a sensation of constant spin. He reported no accompanying symptoms of hearing loss, tinnitus, or aural fullness/pressure.
According to reported symptoms and the results of the initial ENG testing, this patient fit all of the criteria or features mentioned by Lachman and Stahle (1967) for VN. Interestingly, this patient had a similar episode of vertigo approximately one year previously, also without aural symptoms;at that time, he did not feel completely free of symptoms for six weeks. The suspected diagnosis at that time was also VN, though he did not undergo vestibular testing.
Results
The following are the results of vestibular testing during the most acute stage of symptoms. At the end of the results section, Table 1 displays the results of follow-up testing on four separate occasions.
Spontaneous Nystagmus
Practicing audiologists know that the definition of spontaneous nystagmus is not always consistent among authors. Sometimes, spontaneous nystagmus is referred to as gaze nystagmus with eyes closed or as positional nystagmus that occurs in every head position. At our facility, we define spontaneous nystagmus as nystagmus that occurs in the primary position (i.e., sitting, eyes open or eyes closed, and head straight) and positional nystagmus as nystagmus that intensifies or changes direction in other static positions.

Figure 1. Spontaneous Nystagmus
Sitting w/vision: very mild right beating nystagmus (RB). Sitting without vision: 34°/sec RB.
Positional Nystagmus
Figure 2 below shows slow phase velocity values for each static position. There was an average positional nystagmus of 43/sec.

Figure 2. Positional Nystagmus
Even novice clinicians will recognize that this level of spontaneous/positional nystagmus is intense. In fact, it is stronger than the response that would be obtained with caloric stimulation in more than half of the normal population. The clinical finding of spontaneous/positional nystagmus suggests an uncompensated peripheral lesion, typically on the side opposite the direction of the nystagmus (less commonly, irritative vestibular lesions such as active-state Meniere's syndrome can result in spontaneous nystagmus beating toward the involved side). Consequently, as presented in this case, right beating spontaneous nystagmus would imply an uncompensated left peripheral vestibular lesion. If right beating nystagmus is paired with left unilateral weakness, a left peripheral vestibular lesion is assumed.
The tracings above show only the horizontal component of the nystagmus but it is not uncommon to have torsional nystagmus, with a strong vertical component as well. Furman and Cass (2003) provide a good explanation of why patients like this have a horizontal-torsional spontaneous nystagmus:
"The patient's nystagmus is caused by the imbalance in afferent activity from the left and right labyrinths and is probably a result of imbalanced semicircular canal activity rather than imbalance of otolith activity. Because of the orientation of the three semicircular canal pairs, the afferent activity from the intact ear, if unopposed by activity in the affected ear, combines to produce a slow component of nystagmus whose direction has specific characteristics. The unopposed right (non-lesioned) ear, horizontal semicircular canal activity drives the eyes horizontally towards the left, and the combined activity of the two right vertical semicircular canals, that is the right superior and left posterior semicircular canals, causes a slow torsional movement of the eyes, with the upper poles moving towards the left. No vertical component is produced because the right superior semicircular canal tends to drive the eyes up, while the right posterior semicircular canal tends to drive the eyes down, thereby canceling the vertical influences. Thus, the predominantly horizontal-torsional nystagmus suggests a net effect of three unopposed semicircular canals of the patient¡¦s right ear combining to produce a nystagmus whose slow component has a horizontal direction toward the lesioned ear and whose quick component (for which the nystagmus direction is named) beats horizontally towards the intact ear."Note the intense vertical (up-beating) component in the figure below. Looking at the raw data above, you will see that with our patient there was a steady and intense vertical (up-beating) nystagmus paired with the already-mentioned strong horizontal (right-beating) component, suggesting a torsional component, not uncommon in such cases.
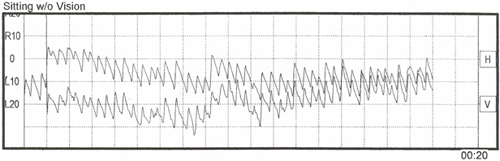
Figure 3. Torsional spontaneous nystagmus (horizontal and vertical) in sitting with eyes closed position.
Gaze Nystagmus
Mild right-beating nystagmus was noted in gaze up and gaze right. No nystagmus was recorded in gaze down or gaze left. Gaze nystagmus is generally considered a finding denoting central vestibular involvement. However, in this case, it is simply a reflection of intensity of the acute peripheral vestibular nystagmus that cannot be totally suppressed with visual fixation. The fact that the patient has more difficulty in suppressing the right beating nystagmus with gaze fixation to the right is known as Alexander's law, (Robinson, Zee, Hain, Holmes & Rosenburg, 1984) which says that the frequency and amplitude will increase when a patient looks in the direction of the quick component of the nystagmus.
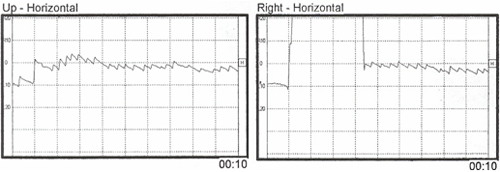
Figure 4. Horizontal gaze nystagmus with gaze 20° up and gaze 20° right.
Smooth Pursuit Tracking
Smooth pursuit tracking was relatively normal, except for an occasional mild over-riding right-beating nystagmus.
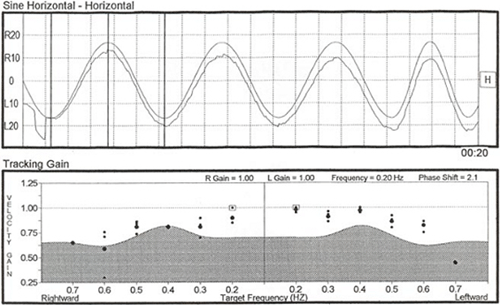
Figure 5. Relatively normal smooth pursuit.
Saccadic Tracking
Computer analysis of saccadic tracking showed normal velocity and accuracy measures, but abnormal latencies and an over-riding right-beating nystagmus (more intense when the eyes were deviated to the right;recall Alexander's law). One has to be cautious that the abnormalities noted here are not attributed to central vestibular pathology, as they simply reflect the influence of the intense spontaneous/positional nystagmus on the ocular motor battery.
Figure 6. Saccadic tracking recording. Note the overlying right beating nystagmus.
Headshake
There was a right beating nystagmus after headshake, which was not unexpected in view of the intense right beating spontaneous/positional nystagmus.
Caloric Irrigations
Due to the intense right-beating spontaneous/positional nystagmus, the results at first glance appear to be rather unusual, and one might mistakenly suspect possible technical error. The computer analysis showed a 57% left ear caloric weakness (although the actual caloric asymmetry is more accurately interpreted as 100%) and a 173% right directional preponderance (DP), indicating the right-beating nystagmus was so strong that the nystagmus direction did not change to left beating at any time, even with cool irrigation to the good right ear. Computer-scored caloric values were as follows (see Figure 7):
Right cool = 8 degrees/sec. (right beating)
Left Cool = 38 degrees/sec. (right beating)
Right warm = 71 degrees/sec. (right beating)
Left warm = 21 degrees/sec. (right beating)
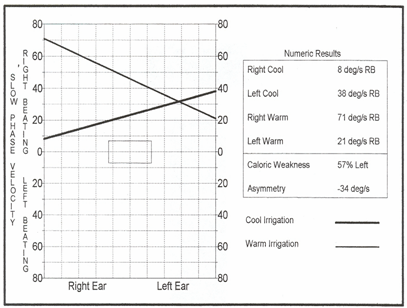
Figure 7. Caloric test butterfly graph showing vestibular asymmetry.
At first glance the caloric values appear inconsistent and unreliable because of the large disparity between the values obtained in the right ear and the fact that all of the calorics measured right-beating nystagmus. However, considering the 40sec right-beating nystagmus already noted in the static supine head elevated position (the specific position maintained during caloric irrigations), caloric results are both meaningful and reliable. To precisely measure what was truly elicited by caloric stimulation, one must add or subtract the pre-existing nystagmus value (in this case 40 sec) as shown below. Remember first what the expected outcome should be with the various caloric irrigations (acronym COWS- Cold opposite, Warm Same):
Right Cool = left beating (LB)
Right Warm = right beating (RB)
Left Cool = right beating (RB)
Left Warm = left beating (LB)
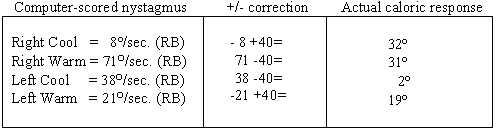
Table 1. Correction factor for caloric responses.
Neither the response from the right cool or the left warm irrigations (both of which should have provoked left beating nystagmus in healthy systems) was strong enough to cancel and then reverse the intense spontaneous/positional nystagmus. Look carefully at the left ear "responses" shown on the pod chart below and you will note that there is no real change in the intensity of the pre-existing nystagmus with either the warm or cool irrigations. That being the case, why did the computer score the left warm as a response of 21 sec and the left cool as 38 sec? Keep in mind that the algorithms for response detection for left warm irrigations are written to detect the strongest left beating response, or the point most closely approximating left beating, in this case a point scored at 21 /sec, but still right beating. Similarly, the algorithms for response detection for left cool irrigations are written to detect the strongest right beating response and in this particular case, a "peak" of 38/sec right beating was selected. Knowing this, experienced clinicians can disregard the algorithms and make appropriate adjustments to the response. Applied here, those corrections now indicate not a 57% unilateral weakness but essentially a 100% unilateral loss.
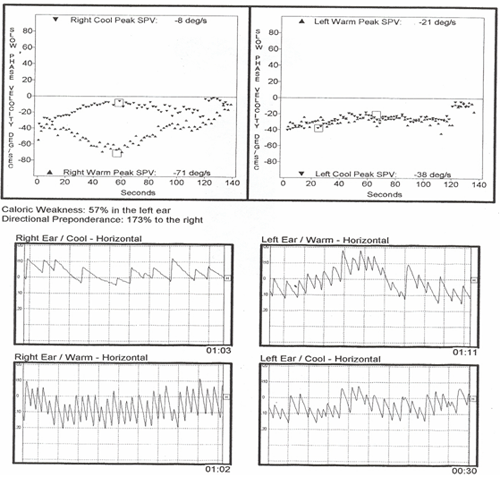
Figure 8. Caloric irrigation pods and raw data.
Audiogram
Audiometric thresholds revealed normal and symmetrical hearing sensitivity bilaterally (Figure 9). Speech reception thresholds were obtained at 5 dBHL bilaterally, consistent with established pure tone thresholds, and word recognition values were bilaterally excellent (100% at presentation level of 35 dBHL). These results were expected;you will recall that he reported no aural symptoms preceded or accompanied the vertiginous episode.
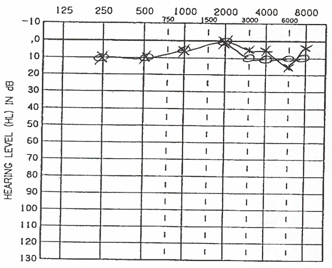
Figure 9. Audiogram depicting normal and symmetric hearing sensitivity.
Computerized Dynamic Posturography (CDP)
A summary of the following CDP results is shown in Figure 10. The abnormal composite equilibrium score indicated significant overall balance impairment. Sensory organization test (SOT) revealed a severe vestibular dysfunction pattern due to falls on all three trials of condition #5 (eyes closed/swaying platform) and one fall on condition #6 (eyes open, swaying platform and swaying visual field). A vestibular dysfunction pattern is common with peripheral vestibular lesions and reflects a marked inability to effectively rely upon vestibular cues for postural control, especially in the absence of adequate visual or somatosensory input (such as when walking in the dark or over uneven surfaces). The motor control test revealed normal automatic postural responses to sudden linear perturbations. Normal motor control test latencies are expected with peripheral vestibular insults. The adaptation subtest was attempted but was aborted after only two toes-up trials, as the patient reported feeling too dizzy and sick to continue.
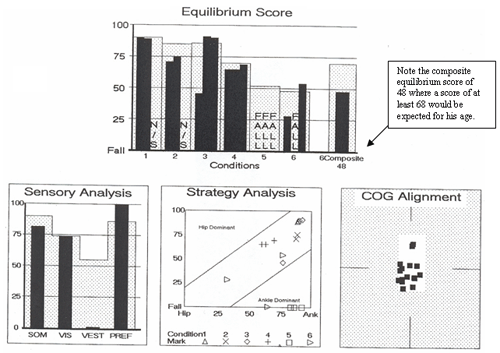
Figure 10. Posturography results revealed a severe vestibular dysfunction pattern.
Rotary Chair
The rotary chair test was unable to be performed because the patient was too ill to tolerate further testing at that time. However, rotary chair testing was conducted on follow-up evaluations;his first rotary chair test results are presented in Table 2.
Follow Up
This patient was asked to return for follow-up testing at 2 weeks, 5 weeks, 10 weeks, and 8 months post acute spell. Follow-up vestibular tests were performed in conjunction with vestibular rehabilitation appointments. Repeat posturography tests were administered by the physical therapist. At each follow-up appointment, an abbreviated ENG was performed (tests included are shown in Table 1). Each successive caloric test still showed a 100% left caloric weakness. The two week post caloric test revealed a 109% DP, which was reduced from the 173% DP at the initial test. The reduction of DP is expected due to the reduction in strength of the spontaneous/positional nystagmus. At the first follow-up appointment, bi-thermal calorics were performed. Only mono-thermal (cool) irrigations were performed on the remaining follow-up appointments. The results of these tests are summarized in Table 2 below.
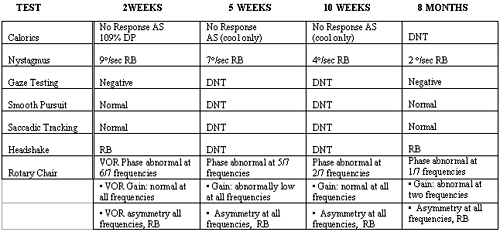
Table 2. Results of follow-up vestibular testing. DNT= did not test;RB= right beating;VOR= vestibular-ocular reflex
Vestibular Rehabilitation
An initial rehabilitation session took place five days after vestibular testing. Functionally, he was unable to perform normal activities such as driving, working, bending over, looking up, or moving his head. He stated that as long as he was sitting he had no dizziness. A neurological workup yielded normal results, but he presented with poor static and dynamic balance, decreased gaze stabilization, and a vestibular dysfunction pattern. He avoided all head movement and held to the walls to walk. Vestibular rehabilitation was initiated to facilitate adaptation through movement. He had been prescribed Valium, but was no longer taking it. He was seen three times before formal discharge from therapy, but was seen two other times for case study follow up. The results of the therapy are summarized in a chart at the end of the results section (Table 2).
Initial Evaluation
Scoring on the Jacobson Dizziness Inventory (Jacobson & Newman, 1990) (see Table 3 for explanation of this and other measures) indicated he was severely destabilized and felt non-functional for daily activities. He was shown how to use surface orientation (see footnote 1) to decrease symptoms, as well as using somatosensation and vision to increase stability. Therapy was started with static standing activities 2 after confirming a positive Romberg, and listing to the left. Gait activities were added to decrease veering and improve mobility. Head movements were started slowly with gait and vestibular ocular reflex 3 (VOR x 1) to increase tolerance to head movements.
Second treatment session
He reported compliance with the home exercise program and showed an improvement with balance and normal activities. He felt as though he was back to 85% of normal. The greatest change was an improvement in his Jacobsen Dizziness Inventory score from 100/100 to 36/100. CDP was normal with a composite score of 80. His home program was changed to narrowing base of support 4 with gait, VOR x 2, more gait accuracy 5, gait on uneven surfaces, sensory integration 6, and visual stimulation 7.
Third treatment session
His perceived return to normal was 95%. He was still having some complaints when backing out his car and walking outside. He had improved gaze stabilization, though it was still slightly below normal with eyes-closed activities, and minimal complaints of dizziness when rolling over. The Jacobsen Dizziness Inventory had improved to 10/100. Functionally, he was able to walk on grass, stairs, jog short distances, drive, and go back to work. CDP was normal with a left posterior center of gravity and composite score of 83. He was discharged to a home exercise program with increased difficulty, adding uneven surfaces 8, including eye-hand sports.
Fourth follow up
His perceived return to normal remained the same. He reported that he had had minimal dizziness while playing with children on the floor. The Jacobsen Dizziness Inventory improved to 2/100, clearly demonstrating improved quality of life. CDP was normal, but showed a slight posterior center of gravity, but not to the left as previously shown, and a composite score of 85. He was to continue with same exercise program, but added habituation consisting of rolling back and forth on the floor.
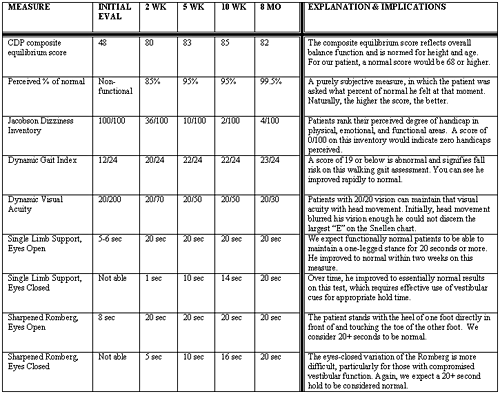
Table 3. Vestibular Rehabilitation: Results and Interpretation.
Summary
We present the following salient points gleaned from this interesting case:
- VN is subjectively and objectively debilitating. The physical, emotional and functional impact of VN cannot be underestimated. Our young, otherwise healthy, and active patient was unable to perform simple daily activities like driving, bending over, looking up or moving his head, let alone fulfill job requirements. This particular patient had spontaneous/positional nystagmus that nearly reached 50¢X/sec. A caloric response of this intensity is considered borderline hyperactive and can induce or provoke extreme vertigo, nausea, and vomiting despite the relatively short duration of the response and its suppressibility using visual fixation. It is no wonder that patients with VN, who suffer unrelenting vertigo for days or weeks and who cannot suppress that vertigo using visual fixation, are incapacitated.
- Physiologic compensation for eye movement (nystagmus) is often prolonged. This patient continued to have a mild degree of nystagmus even eight months later, despite significant functional improvement due to his diligence in completing vestibular rehabilitation exercises. In addition, when a person suffers from a unilateral vestibular loss, recovery of function in the affected ear is not likely, as illustrated in this case by the continued 100% unilateral caloric weakness and residual VOR phase abnormalities despite lack of tonic VOR asymmetry. That is, though central compensation should occur (facilitated by rehabilitation), measurable asymmetry remains.
- The destabilizing effects of VN can last for months. This is especially true if the VN has caused a complete unilateral loss of peripheral vestibular function. As long as the spontaneous/positional nystagmus continues it would stand to reason that some resultant instability will persist. And in fact, these patients will always experience some slippage in their visual field with rapid head movement.
- Patients are generally given vestibular suppressants for their acute complaints, but are often not thoroughly evaluated for vestibular function nor followed for the residual effects of a severe unilateral vestibular deficit. Though compensation can occur with time, our experience has shown that it can be expedited with vestibular rehabilitation directed toward maintenance of stance, return to work, and improvement in active daily living. Unfortunately, most patients with VN do not have timely vestibular evaluation or specialized vestibular/balance rehabilitation, thus delaying or preventing appropriate compensation and improved quality of life. As audiologists, it is our responsibility to educate physicians and other professional colleagues regarding the value of proper functional evaluation and the efficacy of rehabilitation.
- Surface orientation: The use of the ground to orient patient to the world. This can be touching the wall, awareness of the feet flat on the floor, feeling solid on the chair or bed. The surface (ground) makes an individual feel solid and less dizzy.
- Static balance: Standing activities like feet together eyes closed, standing on one leg, standing one foot in front of the other.
- Vestibular ocular reflex: An individual looks at a target and makes small head oscillations keeping the target in focus.
- Narrow base of support: Walking or standing activities with the feet closer together than the normal stance.
- Accuracy activities: Activities that require precision with other movements such as head movements while standing or walking with a narrow base of support.
- Sensory integration: The adaptation of the balance system using somatosensation, vision and vestibular information.
- Visual stimulation: stimulating or trying to irritate the system with visual input such as lights, designs (such as checkerboards), and with objects coming near or towards the face. This is to try to decrease sensitivity to busy environments such as walking in a crowd.
- Uneven surfaces: Activities on foam, half-foam rollers, grass, stairs, inclines, dirt, gravel.
Baloh, R.W., Lopez, I., Ishiyama, A, Wackym, P.A., and Honrubia, V. (1996). Vestibular Neuritis: Clinical pathologic correlation. Otolaryngology Head and Neck Surgery, 114, 586-592.
Coats, A.C. (1969). Vestibular Neuronitis. Acta Laryngologica (suppl), 251, 5-28.
Furman, J.M., & Cass, S.P. (2003). Vestibular Disorders: A Case-Study Approach, 2nd Edition. New York: Oxford University Press.
Jacobson, G.P. & Newman, C.W. (1990). The development of the Dizziness Handicap Inventory. Arch Otolaryngol Head Neck Surg, 116(4), 424-7.
Lachman, J., & Stahle, J. (1967).Vestibular Neuritis. A clinical and electronystagmographic study. Neurology, 17 (4), 376-380.
Leigh, R.J., & Zee, D.S. (1999). Neurology of Eye Movements, 3rd Edition. New York: Oxford University Press.
Robinson, D.A., Zee, D.S., Hain, T., Holmes, A.M., Rosenburg, L.F. (1984). Alexander¡¦s law- its behavior and origin in the human vestibulo-ocluar reflex. Ann Neurology, 16, 714-722.
Ruttin, E. (1909), as cited by Hess, K. (1999). Journal of Vestibular Research 9, 221.
Schuknecht, H.F., & Kitamura, K. (1981). Vestibular Neuritis. Annals of Otology, Rhinology, and Laryngology (suppl), 78(90), 1-19.

