Editor’s Note: This text course is an edited transcript of a live seminar. Download supplemental course materials.
Amy Winston: Today, Dr. Stoner and I will highlight some auditory brainstem response (ABR) cases that we have seen here at Rush. We have a diverse patient population and we see both classic and complex cases. Through an illustration of a few cases, we hope to reinforce what we know and understand about the auditory pathway and ABR testing. Our learning objectives today are to discuss the characteristics of a normal click-evoked ABR relative to the waveform neural generator sites, to discuss how observed changes in the click-evoked ABR waveform can assist in characterizing the nature and degree of identified hearing loss, and finally, to explain the effects of disruptions at specific points along the auditory pathway on the ABR waveform.
Before we begin to discuss cases, we wanted to review what the ABR is, what it is not, the neural generators sites, and the standard waveform characteristics. Since its inception in the late 1960s, the ABR has been an invaluable diagnostic tool for audiologists. As an objective test, it allows us to gain information about the integrity of the auditory system, and provides an estimation of hearing sensitivity for patients who are unable to give us reliable subjective information. This would include infants and young children, but also difficult-to-test older children and even some adults. We should all be clear that ABR is not a direct test of hearing; it is a test of synchronous neural function. However, it can be used to estimate hearing sensitivity, which is how we will be talking about it today.
Click Stimulus
There are different stimuli that we can use with the ABR, the most basic being the broadband click stimulus. The click is not frequency specific. It is thought to be generated in the cochlea in the 2000 to 4000 Hz range. We also can use frequency-specific tone bursts, most commonly 500, 1000, 2000, and 4000 Hz stimuli. These are very brief stimuli that trigger a rapid, synchronous neural onset. The click tends to be a little more efficient than the tone burst stimuli, simply because of its construction. The tone burst can give us a good response, but we get a classic waveform morphology with the click stimulus.
As we go through the presentation, Dr. Stoner and I will be focusing on responses to click stimuli; we did that for a few reasons. The first reason is because of the classic ABR response we can elicit. In addition, there are an abundance of normative data that have been developed using the click. Finally, the majority of cases we will present today were started as follow-up testing to newborn hearing screenings. The automated ABR systems use a click stimulus in their protocol, so we will typically begin our testing with a click stimulus as well, and then proceed with tone burst stimuli as needed from there. Fortunately for us, all of these stimuli can be conducted both by air and by bone conduction to allow for a full investigation of the auditory system.
There are five primary ABR waveform components, waves I through V. We have been able to establish the neural generator sites for these specific wave components. Wave I is generated from the distal portion of cranial nerve VIII where it exits the cochlea. When looking at the waveform, we are then able to follow the pathway up through the lower brainstem. Through replicated research studies, we have a good understanding of when these waveforms should occur post-stimulus onset, known as absolute wave latencies. We also have a good understanding of when these waves should occur relative to one another, known as interwave latencies. Understanding the neural generator sites, latencies and interwave latencies provides a good benchmark against which to measure change, which is what we will be doing today.
Neural Generators
As I mentioned, wave I is initiated at the peripheral or distal portion of cranial nerve VIII; it should occur at approximately 1.5 msec for a click. As we move along cranial nerve VIII, wave II is thought to originate from the more proximal portion of that nerve at about 2.5 msec. Wave III is generated at the level of the cochlear nucleus and should occur at approximately 3.5 msec. Wave IV is generated in the region of the superior olivary complex/lateral lemniscus at 4.5 msec. Finally, wave V is generated in the region of the lateral lemniscus/inferior colliculus around 5.5 msec.
Robin Stoner: When we are conducting the ABR, we are looking for predictable changes in the waveform in response to changes in the auditory stimulus, which may include intensity or polarity. This will provide diagnostically significant information about the presence and type of hearing loss. Some of the waveform characteristics may include the presence or absence of wave V using air- or bone-conducted stimuli, latency measures or changes in the waveform in response to polarity changes, as well as the waveform morphology and the amplitude.
As stated earlier, the ABR is not a true test of hearing, but it can help to estimate the degree of hearing loss by finding the lowest intensity level where wave V is present and replicable. Latency information and results from bone-conducted stimuli can help to provide insight to the nature of the hearing loss. Finally, auditory neuropathy spectrum disorder can be confirmed or ruled out by looking at the polarity effects on the waveform.
Case Studies
The cases that we present today show expected ABR findings with both normal and abnormal auditory function at various anatomical points along the auditory pathway. They illustrate how outer, middle, and inner ear dysfunction as well as auditory neuropathy and brainstem dysfunction influence the characteristics of the click-evoked ABR.
Case #1: Normal Auditory Function
This first patient was a three-year-old male who was first seen in our outpatient clinic for an audiological evaluation. During the case history, his parents stated that their son only responded to certain sounds and had a limited expressive vocabulary. Their primary concern was for his speech and language development. In addition, they mentioned that a psychological evaluation completed one month prior indicated that the patient may have a mild degree of autism. His parents reported that the pregnancy and birth histories were essentially normal. His mother said that she had a C-section because his heart rate dropped, but there were no other complications. In addition, his parents denied a history of ear infections and there was no family history of hearing loss.
The audiological evaluation was completed with two testers and limited results were obtained. We completed behavioral audiometry using visual reinforcement audiometry (VRA); the patient was not developmentally appropriate for conditioned play audiometry even though he was three years old. We obtained a minimal response level at 30 dB at 2000 Hz in the sound field, and a speech awareness threshold (SAT) was obtained at 0 dB. These two values were not in good agreement and were not what we would expect for a three-year-old. Tympanometry was also completed and the results were normal. The patient did not tolerate otoacoustic emissions (OAE) testing. Based on all of this and his history, a sedated ABR was scheduled.
Our first item of business was to run a high-intensity, air-conducted click stimulus and then change the polarity. A robust waveform was noted, and no inversion of the waveform was observed when the polarity was changed. At the high intensity level, the absolute and interwave latencies were within normal limits. A reproducible Wave V was noted down to 20 dB nHL in both ears. The waveforms are shown in Figure 1.
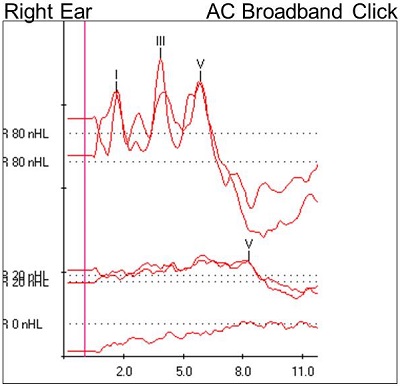
Figure 1. Case #1 ABR results.
At 80 dB nHL, we see nice waves I, III, and V. They are at the expected latencies. We also see at 80 dB that we used a rarefaction click and a condensation click. When we changed the polarity, our waveform was still intact. There was no inversion of the waveform. In addition, we see a well-formed wave V at 20 dB. Finally, the 0 dB run shows no identifiable waveforms, which is what we would expect. Furthermore, it confirms that the 20 dB run and the 80 dB run were true responses.
Case #1 Discussion
Let’s discuss these findings. We have ruled out auditory neuropathy spectrum disorder because the waveform did not invert when the polarity was changed, and auditory function to the level of the brainstem is normal as noted by the normal latency values and the fact that wave V was intact at 20 dB nHL. We know this this to be true for at least a portion of the frequency range of the click stimulus. Keep in mind that the click stimulus is between 2000 and 4000 Hz.
The click was just our starting point. We wanted to know more about this patient’s hearing, so tone burst testing was completed. We used 500, 1000, 2000, and 4000 Hz tone burst stimuli and the results using these stimuli were also normal. Our overall interpretation was a normal ABR study.
The latency-intensity data for this ABR are shown in Figure 2. We would expect to see normal absolute latencies for waves I, III, and V as well as normal interwave latencies. Wave V latency was noted in the normal range, which is the gray shaded area, for 80, 60, 40, and 20 dB nHL (Figure 2, top). The other latency-intensity functions shown are the I-V interwave latency, as well as wave I and wave III absolute latencies. We know that it is normal for waves I and III to disappear as we decrease intensity.
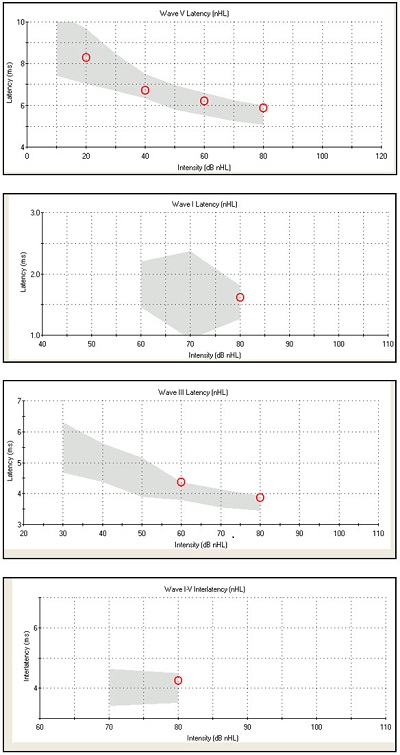
Figure 2. Normative data for Case #1, from top to bottom: wave V latency, wave I latency, wave III latency, wave I-V interwave latency.
Now that you know what normal auditory function looks like, let’s look at a few cases of auditory dysfunction, starting with a case of conductive hearing loss.
Case #2: Conductive Hearing Loss
Amy Winston: This next case is of a three-week-old female. She was seen for an unsedated ABR after failing her newborn hearing screening bilaterally. We know that she was born at 36 weeks and that mom’s health was compromised during pregnancy by prenatal diabetes. Medical history for the patient was significant for a number of things including bilateral microtia, and left external auditory canal atresia and stenosis of the right external auditory canal. So upon visual inspection, we already know that she has certain issues that will contribute to a conductive hearing loss. This patient did have a CT scan prior to arriving at our clinic, which confirmed that there was no left external auditory canal. It also showed other unexpected findings that were very helpful to us during our testing. Ossicular chain malformation was noted in both ears, and it appeared that the malleus and the incus were fused on both sides. This presented additional concerns for obvious reasons, and led us to a continued hypothesis for conductive hearing loss in both ears.
Before we started the ABR, we completed tympanometry using a 1000 Hz probe due to her age, as well as OAEs. The results for tympanometry were grossly abnormal for the right ear and we were unable to test the left ear due to the aural atresia. Distortion-product (DP)OAEs were absent in the right ear and could not be tested in the left ear. This is what we anticipated for the right ear. Based on the CT findings of ossicular chain malformation, the patient’s abnormal tympanometry and absent OAE results were expected.
We started the ABR testing in the right ear. The patient did have a stenotic ear canal, but it was open. We began with an air-conduction rarefaction click and found that waves I, III, and V were present at our starting intensity of 80 dB nHL. Following protocol, we changed the stimulus polarity to condensation. We did not see an inversion of the high-intensity waveform, ruling out auditory neuropathy spectrum disorder. We did note that the absolute latencies of waves I, III, and V were prolonged at this high intensity, meaning that the timing at which each waveform component was present was later than normal. The interwave latencies, however, were retained, and fell within normal limits. Wave V remained intact and replicable down to 70 dB nHL with the click stimulus. We proceeded to test with a 500 Hz tone burst stimulus and found that wave V was intact and replicable down to 60 dB nHL.
Figure 3 shows the right air conduction click results. It looks as if she has a bifurcated wave I at 80 dB nHL. Absolute latencies are pushed out, but the relative interwave latencies were retained within normal limits. Wave V was replicable at 70 dB nHL but disappeared at 60 dB nHL. Choosing this as wave V threshold was relatively easy in this case.
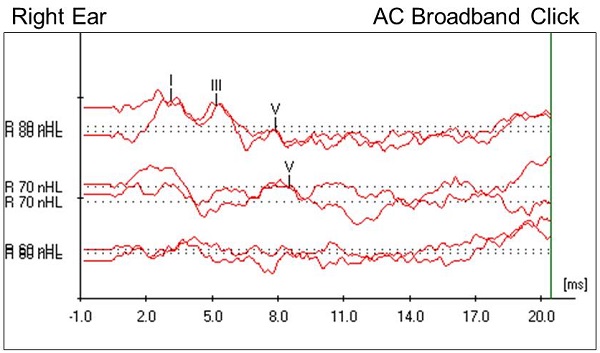
Figure 3. Case #2 right-ear click ABR results.
We know about the right ear, but what about the left ear? Our goal is always to get ear-specific information. Remember that the left ear has no external auditory canal, so testing under insert earphones is not feasible. We moved on to perform unmasked bone conduction testing with the oscillator behind the left ear. We started with a click stimulus, and found that wave V was present and replicable down to 30 dB nHL (Figure 4), which is considered to be within normal limits in our clinic. Bone conduction testing with a 500 Hz tone burst stimulus showed wave V present and replicable down to 25 dB nHL, which is considered normal as well.
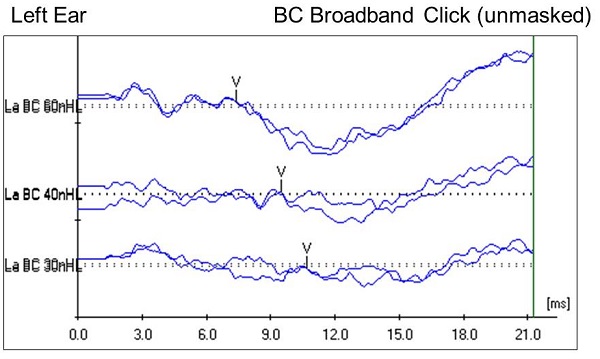
Figure 4. Case #2 left-ear unmasked bone conduction ABR results using a click stimulus.
The left ear was unmasked, but let me clarify how we know this was a left-ear response. The bones of the skull in young children have not completely ossified, and there are additional cartilaginous components within the skull. Because the fontanels have not closed at this age, that alters the normal interaural attenuation for bone conduction; it increases it. Research indicates that unmasked bone conduction testing on a child this age can be done. You can get ear-specific information if you look for certain characteristics of the waveform. Most notably, we are looking for wave latencies that are within normal limits. We found that to be true in this case, which supported our belief that we were looking a response from the left ear, even though it was an unmasked bone conduction response.
Another component that I wanted to note on the bone conduction waveform (Figure 4) is the robust wave I on the high-intensity run, although it is not marked. This lends further support that this is a left-ear response. If it were a response from the contralateral ear, we would be less likely to have such a robust wave I. Bone conduction responses were present and replicable down to 30 dB nHL, which is, again, within normal limits for our clinic.
Case #2 Discussion
Let’s discuss what we know, starting with the right ear. We found prolonged absolute wave latencies, but interwave latencies within normal limits. These two components are indicative of a conductive hearing loss. Remember that we found the click response down to 70 dB nHL and the 500 Hz tone burst stimulus down to 60 dB nHL. We can estimate that there is at least a moderate hearing loss across those test frequencies. It is most likely conductive, based on what we see in the characteristics of the ABR waveform response and what we know about the patient history as well.
In normal hearing individuals, we see the latency of wave V shift out later as the stimulus intensity is decreased; that is essentially what is happening with a conductive hearing loss, just at higher intensities than would be expected. The beauty of the middle ear system is that it gives us a natural boost in intensity of about 60 dB to help overcome the impedance mismatch that occurs as sound waves are traveling quickly and efficiently through the air to the fluid-filled cochlea, which is not an acoustic-friendly environment. The middle ear structures provide a boost in intensity to transfer sound from air to fluid. When we have a middle ear dysfunction, in this case, ossicular chain fusion, it reduces the efficiency of the middle ear transfer function. It reduces the intensity of the stimulus that ultimately reaches the cochlea. Seeing those latencies shifted with a conductive hearing loss makes perfect sense and is exactly what we would anticipate seeing.
In the left ear, because of the characteristics of the unmasked bone conduction response, we do believe that we are seeing normal cochlear function across 500 Hz and some portion of the 2000 to 4000 Hz range. Knowing that this patient has an absent external auditory canal and ossicular chain malformation, we would anticipate a maximum conductive hearing loss in the left ear. Having a normal cochlear reserve would allow her to take advantage of bone-conducted stimuli. She was ultimately fit with a bone-anchored hearing aid (Baha) soft-band device at an outside facility. Moving forward, the goal is to try to obtain as much diagnostic auditory information as possible from the right cochlea.
To summarize, characteristic ABR findings for conductive hearing loss include prolonged absolute latencies. You can see a classic pattern on the normative data graph where all the responses for absolute wave latencies are shifted above the shaded area (Figure 5, top). You will also see interwave latencies within normal limits (Figure 5, bottom). Your bone conduction results should also be within normal limits, which would reflect a normal cochlear reserve. The degree of loss would clearly be reflected by the air conduction wave V threshold, which we would anticipate would be elevated.
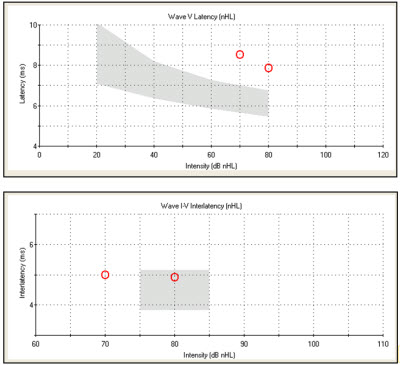
Figure 5. Case #2 latency-intensity function (top) and interwave latency data for the right ear (bottom).
Case #3: Sensory Hearing Loss
Robin Stoner: Our next patient was a nine-month-old female who failed the newborn hearing screening and was referred to us from an outside facility. Her mother accompanied her to the appointment and stated that she felt the patient could hear and was babbling. The mother did not have any concern about the child’s overall development, and we think that is part of the reason that it took so long for this family to follow up. In addition, her mother reported an unremarkable full-term pregnancy, and there were no complications associated with the patient’s birth. A family history of hearing loss was denied, as was a patient history of ear infections. There were essentially no risk factors for hearing loss identified from the case history. It is our clinic’s policy to perform an audiological evaluation with two testers in our outpatient clinic before proceeding to the sedated ABR.
During this patient’s audiological evaluation, we were able to obtain an SAT in both ears using insert earphones. The SAT was 30 dBHL for the right ear and 50 dBHL for the left ear; we would expect to see an SAT around 10 to 15 dB for a nine-month-old. We attempted tonal stimuli but could not obtain any responses due to patient fatigue. We were able to complete tympanometry and acoustic reflex screening using a 1000 Hz ipsilateral stimulus; the reflex was intact in both ears. We tried OAEs, but the patient was too noisy. Based on these results and the patient’s history, we decided to proceed with the sedated ABR. We obtained responses for both ears, but I am going to concentrate mostly on the findings from the right ear.
Once again, we started with a high-intensity, air-conducted click stimulus, and we changed the polarity from rarefaction to condensation. A beautiful waveform was noted at 80 dB nHL (Figure 6). There was no inversion when the polarity was changed. Returning back to the rarefaction click stimulus, we were able to track wave V down to 50 dB nHL; no response was observed below that. We found normal absolute and interwave latencies using the high-intensity click stimulus. The wave V latency was slightly prolonged as the intensity decreased. No response was noted at the output limits (55 dB nHL) of the bone conduction oscillator.
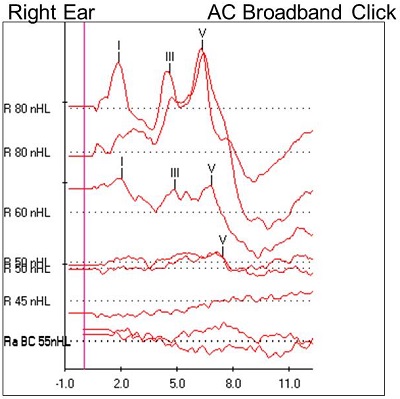
Figure 6. Case #3 right-ear ABR results.
Case #3 Discussion
The presence of a normal waveform at a high-intensity is consistent with the cochlear site of lesion, and it reflects that the dysfunction within the auditory pathway is peripheral to the generator sites of the ABR waveform. In this case, air and bone conduction ABR results were consistent with a moderate sensory hearing loss for a portion of the frequency range of the click stimulus. In addition, tone burst stimuli were utilized to estimate frequency-specific thresholds and define the configuration of the hearing loss. The results indicated a mild sloping to moderate sensory hearing loss. Remember that the ABR is not a true test of hearing, but an objective measure of auditory function.
The sensory nature of this hearing loss is supported by normal absolute wave and interwave latencies at a high intensity, an elevated wave V threshold, and the absence of a response to the bone-conducted stimulus. This patient was ultimately fit with binaural amplification at an outside facility. Since a hearing loss was identified through the sedated ABR, we recommended audiologic monitoring for this patient. Our goal now is to obtain ear- and frequency-specific threshold information for both ears using behavioral test measures, recognizing that it may take a few appointments to get a complete picture of her true thresholds. She did return to the clinic two months after our sedated ABR, and VRA was performed using insert earphones. The results at that time were consistent with the ABR results.
In summary, sensory hearing loss is characterized by wave I falling outside the normal latency-intensity function. Wave V latency is normal at higher intensities, but as the intensity is decreased, it will be prolonged, showing an L-shaped latency-intensity function (Figure 7). The interwave latencies are either normal or shortened. When we are looking at degree of hearing loss, we are looking at where wave V threshold falls. With sensory losses that threshold would be elevated.
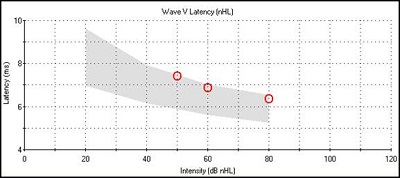
Figure 7. Latency-intensity function for sensory hearing loss.
Case #4: Auditory Neuropathy Spectrum Disorder
Amy Winston: I want to highlight an interesting case of auditory neuropathy. There is still some debate about precisely where the site of lesion is for auditory neuropathy, and we will discuss that as well.
This patient was a three-month-old female when we saw her at Rush for an unsedated ABR. She had some unfortunate social and family dynamics going on at the time. She was brought in by her maternal grandmother, who was unable to give us any information about the baby’s mother, who was her daughter. There was no pregnancy history or information about the mother’s health. The grandmother was also unable to give us any information about this baby’s father, so we did not have a lot of background information. Thankfully, we did have paperwork that the grandmother had brought with her from Cook County Hospital, which is next door to us, indicating that the baby was being followed there by a high-risk team, so we could obtain some information from the physicians there.
We know that she failed her automated ABR newborn hearing screen bilaterally at Cook County Hospital. Furthermore, reports indicated that the baby’s history was significant for a G6PD deficiency. I had not heard of that before, but through some research, I discovered that G6PD is an X-linked enzyme deficiency, specific in the glucose 6 enzyme– phosphate dehydrogenase. Because this is an X-linked deficiency, it occurs primarily in males, and it will be most significant in its presentation in males; however, it can occur in females. Generally the presentation is mild. There are rare instances, though, where additional mutations occur and allow this deficiency to present in a more robust form. As you will see as we go forward in this case, it may be that our patient, unfortunately, fell into this small category of females.
G6PD deficiency causes red blood cells to break down prematurely, which results in anemia, which can be particularly severe in newborns. Secondarily, these patients will then develop jaundice, which we know is a red flag for hearing loss. As the red blood cells break down, bilirubin is developed as a by-product. With the ongoing breakdown of red blood cells, increasingly high levels of bilirubin begin to build up in the blood. When the bilirubin levels are extremely high, the bilirubin is able to cross the blood-brain barrier and go into the brain tissues. The concern is that there may be additional damage within the brain, leading to a condition called kernicterus. Kernicterus includes brain damage, specifically to the brainstem nuclei and the cerebellum.
The auditory pathway encompasses many brainstem structures and the cerebellum as well. The cerebellum is also a critical component in integrating inputs to our balance system. We certainly would not be surprised to see hearing loss and balance problems in patients with cerebellar and brainstem damage.
When the bilirubin gets extremely high, the physician’s concern is to bring the levels back down as quickly as possible. This is typically achieved by an exchange transfusion, because that is the fastest way to clean the blood. On our pediatric intake form, we specifically ask, not only about jaundice, but about exchange transfusions. This gives us an indication of the level of the bilirubin that might have been present. In the notes that the grandmother brought, the physician did indicate that our patient had hyperbilirubinemia to such an extent that she did require an exchange transfusion.
Before we started the ABR, we did our initial testing, tympanometry and OAE testing. Tympanometry with a 1000 Hz probe was normal bilaterally. We found that DPOAEs were absent in both ears across the test frequencies. With the concern of the hyperbilirubinemia and the exchange transfusion, now we were seeing an indication that there was likely some outer hair cell dysfunction. There was great concern going forward as we started the ABR.
In our traditional form, we began the ABR with a high-intensity, rarefaction click stimulus. Per our protocol, the second run was changed to a condensation click stimulus. When I did that, it showed a complete inversion of the waveform. I now had a great concern that this was auditory neuropathy. I did proceed at that point with an alternating click stimulus, which eliminated the waveform entirely.
The waveforms for both ears for this patient are shown in Figure 8. You can see how we separated the rarefaction click and the condensation click. Below those waveforms is the alternating click recording, followed by a 0 dB recording, which looks much like the alternating click run. You can see that the rarefaction and condensation runs are essentially a mirror image of each other.
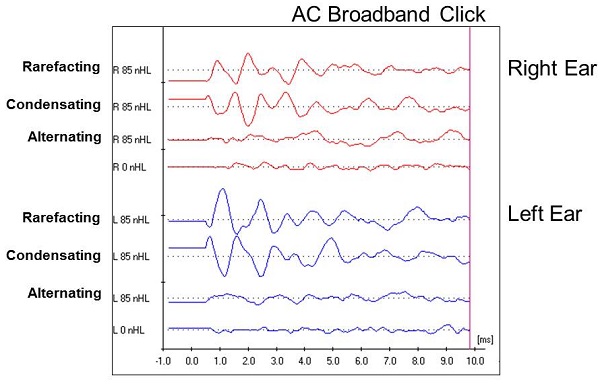
Figure 8. Case #4 waveforms for right and left ear using rarefaction, condensation and alternating click stimuli.
With a normal ABR, we would expect to see a cochlear microphonic prior to wave I. We know the cochlear microphonic is generated within the cochlea, and it will also respond to a change in stimulus polarity. We anticipate the pre-neural cochlear microphonic to invert from rarefaction to condensation click in any patient, but the rest of the waveform should remain intact. You might see a slight latency shift, but you certainly should not see the inversion that we see in this case (Figure 8). Given that the entire waveform inverted, it indicates that this response is coming from the cochlea and does not reflect any kind of neural response. Given this finding, we now have to recognize that we cannot get any information about the integrity of the auditory pathway. Furthermore, it eliminates our need pursue any decrease in intensity to see what we might find, because we are not getting a response from the auditory system beyond the cochlea.
Case #4 Discussion
The classic ABR finding for auditory neuropathy is the inversion of the ABR waveform in response to a change in stimulus polarity. The site of lesion for auditory neuropathy spectrum disorder is not definitively known. At present, there are two schools of thought. One theory suggests that the issue is presynaptic, and the other suggests that it is postsynaptic.
Presynaptic problems would occur at level of the inner hair cell, specifically as an abnormality of the inner hair cell or disruption between the connection between the inner hair cell and the overlying tectorial membrane. It also might be the result of difficulties in the synapse between the inner hair cell and a dendrite of the afferent nerve fiber. All of these problems are going to occur before this becomes a neural response, hence, “presynaptic.”
Postsynaptic issues would include abnormalities of cells on the afferent nerve fibers, which would include dendrites, axons in the afferent nerve fiber, or even the loss of myelin surrounding the nerve fiber, which would certainly disrupt the transmission of the message along the auditory pathway.
In trying to isolate the cause of auditory neuropathy as presynaptic or postsynaptic, some of the literature looks at how well some of these children function when they receiver a cochlear implant. Children with auditory neuropathy, in large part, do not respond well to traditional amplification. In certain cases, some children respond more appropriately with a cochlear implant. So why do some children with auditory neuropathy thrive with a cochlear implant and others do not? The thought there is that perhaps those who do very well have a presynaptic issue, which then would not prevent them from taking full advantage of the cochlear implant. The group that does not do quite as well may suffer from a postsynaptic lesion. These are interesting thoughts and points that you might want to look at if you are interested in auditory neuropathy.
Going back to our patient, hyperbilirubinemia is certainly a risk factor for hearing loss, but it is a major risk factor for auditory neuropathy, specifically. Our patient was a little bit different than the classic case of auditory neuropathy because her OAEs were absent. For the majority of cases involving auditory neuropathy, the literature suggests that OAEs are most often present, particularly when children are very young. The fact that we did not have DPOAEs in this case suggests that there was likely outer hair cell dysfunction and possibly a sensory loss in addition to the auditory neuropathy. I mentioned that our patient underwent an exchange transfusion, so there is a concern of kernicterus and the accompanying brain damage that could possibly occur. The high-risk team involved in this patient’s care had not yet completed all their evaluations, but concern was expressed about some possible brain damage.
There were some different thoughts when it came to recommendations and a future treatment plan. One recommendation was to proceed with the rest of her comprehensive evaluations, and in the future, we could consider cochlear implantation. Some literature suggests that patients who develop auditory neuropathy secondary to hyperbilirubinemia will recover some neural function. In these instances, an ABR at a later date will show recovery and look like a normal ABR waveform. Obviously the global issue we face is the timing of implantation – is it urgent or do we watch and wait? There is a bit of a tug of war at times with some of these patients. We would recommend, and we did recommend, additional ABR testing going forward. We do not assume from the first test that auditory neuropathy will be permanent. We want to evaluate her in another six to twelve months and repeat the ABR.
Case #5: Brainstem Dysfunction
Robin Stoner: This is a case of brainstem dysfunction in a two-month-old female. She arrived to our outpatient clinic via medical transport from her long-term care facility. She was accompanied by her nurse and respiratory therapist. We generally do not see medically involved cases at such a young age in our outpatient clinic, which caught my attention. Her mother was not at the appointment, but from the electronic medical record and the physician’s referral, we knew that the patient did not pass the newborn hearing screening, which was the reason for the appointment. We also knew that she was born at 38 weeks with a normal birth weight. The patient’s mother was under the care of a high-risk fetal neonatal center as well. Her medical history was quite involved and included myelomeningocele, Chiari II malformation, perinatal intraventricular hemorrhage, ventriculoperitoneal (VP) shunt, gastrostomy tube (G-tube) and central apnea. An MRI of the brain was completed during her stay in the neonatal intensive care unit (NICU) at Rush. It revealed no recognizable fourth ventricle, although it appeared to show a remnant of the upper cervical cord. A physician’s note also indicated that the cerebellar tissue was herniated into the upper cervical spinal canal, and the cerebellar tissue appeared to wrap around the medulla and the upper cervical cord. In addition, the patient had central apnea, which required long-term ventilation and tracheostomy.
Using an air-conducted click stimulus at a high intensity, we observed a clearly identifiable wave I. There was no inversion of wave I when the stimulus polarity was changed. The absolute latency of wave I was within normal limits at all of the tested intensities. Wave I remained intact down to 20 dB nHL for both ears. However, waves II through V were completely absent at all intensities for both ears. Waveforms are shown in Figure 9.
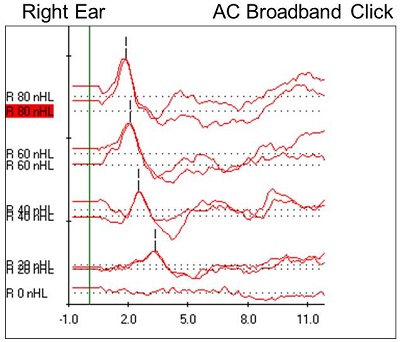
Figure 9. Case #5 right-ear ABR results.
Right away, we can see a very robust wave I at all intensities, with no inversion present at 80 dB nHL. We can also see that there is nothing identifiable after wave I. I wanted to do a 0 dB run to rule out artifact or external noise, and we saw a flat line, meaning that the wave I responses were, in fact, neural responses. It is interesting to note also that, generally, wave V appears at lower intensities and all the earlier waveforms disappear; in this case, we had a robust wave I down to threshold. Figure 10 shows the latency-intensity function for wave I. At the high intensity, it falls within that normal shaded region, and it shifts appropriately as we decrease the intensity.
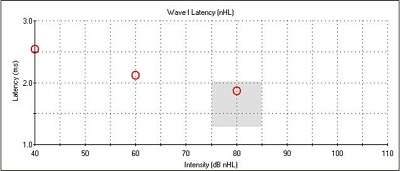
Figure 10. Latency-intensity function for wave I of Case #5.
The patient was still asleep, so we performed 1000 Hz tympanometry. The tracings were grossly normal, meaning that we saw a nice peak. With the 1000 Hz probe tone, you are not looking at the standard values associated with the classifications of Type A, B, C, et cetera. If the tracing is flat, then we know it could be consistent with middle ear dysfunction. However, when we see a nice peak tracing with a child this young, it is either normal middle ear function or possibly some effect from the ear canal. We were able to perform TEOAEs and DPOAEs, and they remained intact in both ears, confirming normal middle ear function from the tympanometry as well.
Case #5 Discussion
Let’s tie her history into the ABR, tympanometry, and OAE results. Myelomeningocele is neural tube defect and the most common type of spina bifida. It is a condition in which the back bone and the spinal canal do not close before birth (Foster, Kolaski, & Riley, 2012). Chiari II malformation is a congenital malformation of the brain that is nearly always associated with myelomeningocele. Significant manifestations of Chiari II malformation include structural changes to the pons and the fourth ventricle, and downward displacement of the medulla, fourth ventricle and cerebellum into the cervical spinal canal (Incesu, Khosla, & Aiello, 2011). The automated ABR used for newborn hearing screenings looks for wave V, not wave I, so we know why she referred on the hearing screening in the NICU. Recall that wave I corresponds to activity from the peripheral portion of the VIIIth cranial nerve. In addition to that, OAE results were consistent with normal outer hair cell function and support the ABR results.
Neurologic evaluations are typically conducted with patients like this to determine the status of nerve-related functions below the defect. The diagnostic ABR allows us to look at the entire waveform, and we are able interpret which waves are present or absent. This ABR did reveal a clearly identifiable wave I only. The ABR results for this patient indicate that the generator sites for wave I are functioning normally, but things start to go awry as early as the proximal portion of cranial nerve VIII. Recall that the patient’s history included no recognizable fourth ventricle on the MRI study; the fourth ventricle is located within the pons or in the upper part of the medulla. The superior olivary complex, lateral lemniscus and the inferior colliculus are also located in the pons. The generator sites for waves IV and V are located in the pons. Our ABR findings are consistent with the patient’s MRI findings, which indicated no fourth ventricle.
What do you do when you get a case like this? My first thought was to go to the literature, and my second thought was to tell all my audiology friends. Hall (1992) notes that gross ABR abnormalities, like the ones we saw on this patient, are usually found in patients with mesencephalic/pontine or lower central nervous system clinical signs. In terms of recommendations and treatment, she was not a traditional candidate for a cochlear implant or a brainstem implant. We recommended that she follow up with the referring physician, who was her pediatrician, and we would hope that she is under the care of a neurologist and that she is also enrolled in early intervention.
What could be a possible communication mode for a patient with such as compromised central system? At this point, we may not know that answer. She was only two months old and we did not know if any other sensory systems had been evaluated. A referral to ophthalmology was recommended. Pending the outcome of other sensory evaluations, we recommended investigating the use of visual or tactile communication strategies.
Questions and Answers
With the Case #5, would you assume that that there is no use of auditory information with the central pathology?
Honestly, we do not know. Because of her age, it is difficult to predict what is going to happen or what information she can utilize. Further evaluation, including ABR, would be in order. To date, we have not seen her back.
What is the time allotted for testing sedated and unsedated ABR?
In our clinic, our natural-sleep ABRs are scheduled for two hours. By the time the baby is fed, burped and changed, it takes a good chunk of time before we even complete testing, which can take all of the two hours. The sedated ABRs are a bit different. We schedule those for an entire morning. They are performed in our pediatric intensive care unit (PICU). We have a whole anesthesiology team and a nurse who is assigned to us as well. We have the patient come in early in the morning, because with sedation, they cannot eat or drink anything prior to the testing, so the earlier, the better. They come in early and are usually released about noon.
How do you describe ABR results to parents if the ABR is not a true test of hearing?
We try to give parents some understanding about what we are doing and what the test measures. The ABR does give an estimation of the sensitivity of sound based on responses from a good portion of the auditory system. However, we do use the term “estimated hearing loss” when counseling on results that are consistent with hearing loss. We use normative data to make those estimations, but it is not a precise threshold like what we obtain with subjective testing.
References
Foster, M. R., Kolaski, K., & Riley III, L. H. (2012). Spina Bifida. Retrieved from https://emedicine.medscape.com/article/311113-overview
Hall III, J. W. (1992). The handbook of auditory evoked responses. San Diego, CA: Singuluar Publishing.
Incesu, L., Khosla, A., & Aiello, M. R. (2011). Imaging in Chiari II malformation. Retrieved from https://emedicine.medscape.com/article/406975-overview
Cite this content as:
Winston, A.K., & Stoner, R.B. (2013, November). ABR: An illustration of auditory dysfunction through clinical cases, presented in partnership with Rush University. AudiologyOnline, Article 12179. Retrieved from: https://www.audiologyonline.com

