The current criterion for implant candidacy has evolved since the initial Food and Drug Administration (FDA) approval of single channel implants in 1984, and multi-channel devices in 1985. At that time, candidacy was restricted to adults only with profound sensorineural loss and speech recognition of less than 10% in the best-aided condition. In a report to the advisory council of the The National Institutes on Deafness and other Communication Disorders, Dr. Ralph Naunton, Director of Human Communications, May 7, 1998, reported that, "...the benefits of cochlear implants are not limited to speech recognition but extended into dramatically improved language learning and language skills" (Tucker, 1998, p. 3). Since that report, implant criteria have expanded, allowing a greater segment of the hearing impaired population to take advantage of the technology. Implants are now approved for both adults and children 12 months and older. The current criteria used for assessing candidacy vary slightly between implant manufacturers. The variation in criteria is related to each manufacturer's original submission to the FDA for device approval. In general, the criteria are as follows (adapted from the Cochlear Americas web site (2005)):
Adults:
- Severe to profound hearing loss bilaterally
- Little or no benefit from hearing aids, defined as speech recognition scores of less than 50% on sentence level testing in the ear to be implanted and less than 60% in the non-implanted ear or in the binaural condition
- Severe to profound loss bilaterally
- Little or no benefit amplification
- Lack of progress in the development of auditory skills
- High motivation and realistic expectations regarding benefit from the child and parents
- Profound sensorineural hearing loss bilaterally
- Lack of progress in development of auditory skills with hearing aid(s) or other amplification
- High motivation and realistic expectations from family
- Other medical conditions, if present, do not interfere with cochlear implant procedure
The expanded criteria have led to research questions centering on advanced uses of the technology. Specifically, could an implant benefit other users previously not considered to be a candidate? A group that was felt to be underserved with conventional amplification were those patients with the following audiometric profile:
- Severe to profound high-frequency sensorineural loss (see Figure 1)
- Speech recognition scores in the best aided condition better than or equal to 10% but less than or equal to 60% on CNC words in the ear to be implanted
- Speech recognition scores of less than or equal to 80% for CNC words in the contralateral ear
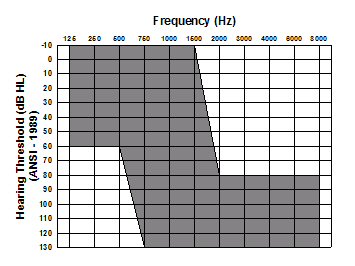
Figure 1. Threshold range for severe to profound, high-frequency sensorineural hearing loss. Note. Figure used with permission of Cochlear Corporation (A. Parkinson, personal communication, March, 2006).
Typically, these patients perform adequately in quiet, relying on their residual low-frequency hearing. However, as the listening environment degrades, communication becomes more difficult due to the lack of high-frequency speech information. Amplification for this group of patients is mostly provided below 1000-1500 Hz, as the severity of the hearing loss above this frequency range renders conventional hearing aids ineffective. The damaged hair cells and/or nerve fibers in these so called high-frequency "cochlear dead regions", will not be sufficient to provide usable acoustic information secondary to the degree of hearing loss (Summers, 2004).
Minimizing Risk for Damaging Low-Frequency Hearing
Cochlear implantation in these patients may be effective; but there is still a significant risk of damaging the residual low-frequency hearing from the insertion of the electrode array or from trauma related to the surgery. Roland, (2002) characterized the damage that can result from placement of the electrode into four separate categories:
- Trauma related to the cochleostomy: The cochleostomy (the opening into the round window in which the electrode is inserted) can result in complete or partial loss of perilymph fluid. Introduction of bone dust from the cochleostomy into the scala tympani can result in the production of new bone formation within the cochlea.
- Fibrous tissue formation within the cochlea.
- Kinking of the electrode during insertion can result in a fracture of the osseous spiral lamina
- Insertion trauma: Damage to the basilar membrane or osseous spiral lamina can occur, which will result in the loss of spiral ganglion cells.
The dilemma that arose was how to insert an electrode that can stimulate the damaged high-frequency fibers within the cochlea without damaging healthy low-frequency fibers. The initial step in this process began with an improvement in surgical technique. "Soft surgical" techniques that focus on minimizing the forces imparted on the cochlea are now being investigated. For example, Cochlear Corporation is now experimenting with an "Advance off Stylet" (AOS) electrode insertion tool (Roland, 2005) that can achieve full insertion (up to a depth of 450 degrees), without contacting the lateral wall when used in conjunction with fluoroscopic visualization of the electrode during surgery. Roland (2005) conducted histologic evaluations of temporal bones that had electrodes placed using the AOS technique inserted using fluoroscopic visualization. He found no trauma to the spiral ligament, basilar membrane, osseous spiral lamina or modiolus. Additionally, the electrodes were found to be in a perimodiolar position within the scala tympani throughout the length of the insertion and intra-cochlear hydraulic pressure was reduced relative to a standard insertion.
Overcoming the surgical trauma issues was a significant hurdle in the treatment of the profound high-frequency loss group. The next step required a re-design of the electrode array. The new array needed to be "minimally invasive" yet able to provide frequency specific stimulation to the high-frequency fibers necessary for improved speech understanding. The final step would be to combine the electrical stimulation presented within the cochlea and the acoustic stimulation presented through the normal sound conducting mechanism - cochlear implant / hearing aid hybrid technology.
Hybrid Technology
Hybrid or Electric-Acoustic Stimulation (EAS) is the merger of these two technologies and is currently in clinical trials in the US. The feasibility of EAS was first reported by von Ilberg et al. (1999). Current studies have shown that acoustic hearing can be preserved in 75 to 90 percent of patients in whom a 20 mm electrode is inserted into the cochlea (Dorman & Wilson, 2004). These authors hypothesized that:
...auditory nuclei in the brainstem, which sort signals from noise, recognize patterns of neural discharge that are unique to acoustic stimulation. The output from even a small region of normal hearing may engage these nuclei in a way that electrically evoked patterns cannot, thereby allowing more of the signal to reach higher levels of auditory processing. Thus the combination of electric and acoustic stimuli can have a synergistic effect on speech understanding, especially in noisy environments. (p. 436)The promise of successful rehabilitation in these patients has not escaped the implant manufacturers. The clinical trials that are now under way in the US are using a combination of currently available and/or modified technology to achieve EAS stimulation. A synopsis of how each company is approaching this goal follows:
Hybrid Technology - Internal Components
Cochlear Corporation: Electrode design techniques that are being investigated minimize trauma to the cochlea and preserve residual hearing by using a short electrode array (10 mm) composed of 6 half band electrodes to ensure that the electrodes are as thin as possible. This electrode is significantly shorter than the standard 24-channel electrode in which the active portion of the electrode is 15 mm in length.
Med-El Corporation: The Flex EAS has an array where the five most apical electrode contacts are single, whereas the basal seven contacts are paired. The intracochlear lead has an overall length of 31.5 mm. Electrode contacts are distributed over a range of 26.4 mm, with the most apical contact being placed 1.2 mm from the tip of the body. To increase flexibility of the new electrode, the five most apical contacts are not paired, through which the diameter at the tip is oval and reduced to 70% of the standard C40 electrode to provide better apical flexibility.
Advanced Bionics: Currently evaluating two slim electrodes that have been found to be less invasive and have shown no signs of intra-cochlear trauma. The "Thin Lateral" and "Helix II" arrays have an insertion depth of 368 degrees for the Thin Lateral electrode, which is designed to approximate the lateral cochlear wall, and 436 degrees for the Helix II electrode, which occupies a more medial position in the scala tympani (Wright, Roland & Kuzma, 2005).
Hybrid Technology - External Components
Cochlear: Combined stimulation is achieved through the use of its current Freedom speech behind-the-ear processor unit in conjunction with a conventional in-the-ear hearing aid. This delivers electric and acoustic stimulation to the same ear, respectively. Patients are also fitted contralaterally with a standard behind-the-ear hearing aid for additional acoustic stimulation.
Med-El: The external processor, DUET is a speech processor/hearing aid combination unit. The acoustic and electric stimulation functions of the system share a common microphone and power supply. DUET processes the sound signal so that it is heard using the ideal combination of natural hearing and cochlear implant hearing. DUET also features a common audio input to connect external devices such as FM systems and MP3 players.

Figure 2. Med-El DUET EAS Hearing System. Note: Figure is from Med-El web site at www.medel.com/ENG/INT/20_Products/25_DUET/999_eas.asp
Advanced Bionics: An external speech processor, currently in development, is reported to include a built in hearing aid.
Hybrid Performance Results
These devices are still in clinical trials in the United States and so performance data with these devices are scarce and only preliminary. Published data form studies conducted in Europe and available through the manufacturers reveal that patients perform significantly better on tests of speech in noise in the EAS condition, compared to the implant alone or in the hearing aid alone condition (see Figure 2). It was further reported that these patients report higher sound quality and improved melody recognition when listening to music in the combined electric and acoustic modality (Kiefer, et al., 2005).
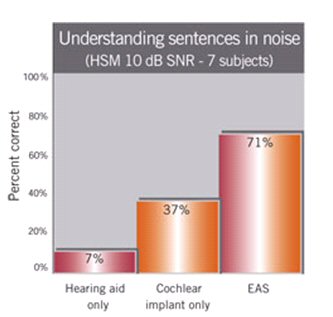
Figure 3. Results from EAS research. Note: Figure is reproduced from Med-El web site at www.medel.com/eng/int/30_advanced_topics/999_eas_results.asp
Additional Risks
Problems associated with successful EAS are not minimal. As previously discussed there is a risk of permanent irreversible damage to residual hearing fibers from the surgery and placement of the electrode itself. A larger long-term concern is associated with future changes in hearing in the implanted ear. Specifically, should the patient experience additional hearing loss, will they need additional surgery using a longer (standard) electrode?
Summary
Expanding criteria have led to an increased number of patients who can now benefit from cochlear implantation, specifically the use of cochlear implant / hearing aid hybrids. The implant pool has expanded as efficacy of the implant has been proven. With modifications to the implant and external sound processors, successful aural rehabilitation to patients who were considered poor implant candidates are now able to be achieved without the risk of loss to residual hearing.
About the Author
Myles Kessler, Au.D. is the director of Audiology and Cochlear Implant Services for a large ENT and Audiology practice in New Haven, CT. Dr. Kessler has had the opportunity of working with every cochlear implant device that has been investigated since the original House 3M single channel implants were first introduced in the eighties. He is a contributing editor to Audiology Online in the area of Cochlear Implantation.
Works Cited
Cochlear Americas. (2005, March 18). Candidacy criteria. Retrieved March 17, 2006, from www.cochlearamericas.com/378.asp
Dorman, M.F., Wilson, B.F. (2004) The Design and Function of Cochlear Implants. American Scientist, 92(5), 436. Retrieved March 15, 2006, from https://www.americanscientist.org/template/AssetDetail/assetid/35121
Kiefer, J., Pok, M., Adunka, O., Stürzebecher, E., Baumgartner, W., Schmidt, M., Tillein, J., Ye, Q., Gstoettner, W. (2005). Combined Electric and Acoustic Stimulation of the Auditory System: Result of a Clinical Study, Audiol Neurotol, 10, 134-144
Roland, P. (2002, November) Electrode Insertion Trauma. Presentation given during the Hearing Preservation workshop at the Indiana University School of Medicine, Indianapolis, IN.
Summers, V. (2004) Do tests for cochlear dead regions provide important information for fitting hearing aids? The Journal of the Acoustic Society of America, 115(4), 1420-1423.
Roland, J.T. (2005). Characterization of Contour Advance electrode insertion. Nucleus Report, May/June. Cochlear Corporation, Denver, CO.
Tucker, B.P. (1998). Cochlear Implants, A Handbook. Jefferson, NC: McFarland & Company.
von Ilberg, C., Kiefer, J., Tillein, J., Pfenningdorff, T., Hartmann, R., Sturzebecher, E., Klinke, R. (1999). Electricacoustic stimulation of the auditory system: New technology for severe hearing loss. ORL: Journal for OtoRhinoLaryngology and Its Related Specialties, 61, 334-340.
Wright, C.G., Roland, P.S., Kuzma, J. (2005). Advanced Bionics Thin Lateral and Helix II Electrodes: A Temporal Bone Study. Laryngoscope, 115(11), 2041-2045.

