Editor's note: This course is an edited transcript of an AudiologyOnline live webinar. Download supplemental course materials.
Introduction
We will start today with a historical overview of electrocochleography (ECochG). We will quickly review of the generators of ECochG. That will lead into a discussion of a clinical test protocol for ECochG. You could set up your ABR equipment using different parameters, and we will talk about why you would want to do that.
The discussion of the test protocol will emphasize one specific aspect of the protocol: electrodes. That that is the most important parameter in all of ECochG test battery parameters. We are only going to spend a moment reviewing the clinical applications of ECochG in adults and focus more of our attention on children, specifically the diagnosis of auditory neuropathy spectrum disorder (ANSD). Then we will wrap up with questions and answers.
History
A well-known auditory neurophysiologist by the name of Glen Wever discovered the ECochG in 1930. He had a great impact on hearing science, including the work done later by Hallowell Davis. As you may know, Hallowell Davis is the father of auditory evoked responses. At Princeton, Wever and Bray (1930a; 1930b) recorded auditory activity from an anesthetized cat. Using an oscilloscope, amplifiers and electrodes, they were able to record activity in the cochlea and from the auditory nerve.
According to the story, they presented words from a speaker during the recordings. The words went from the loudspeaker into the cat’s ear, and through the electrodes sitting on the auditory nerve, they were able to decipher some of what was being said through the loudspeaker. In other words, the cat’s ear was functioning almost like a microphone, hence, the term cochlear microphonic (CM).
Figure 1 shows a timeline of the history of the ECochG. Soon after the discovery of the CM in the cat, the work was published in Science, which is the most prestigious journal in the scientific arena. People all over the world immediately read their work and started to replicate their techniques in humans. Hallowell Davis, in particular, discovered that there was more to it than just the CM and auditory nerve activity. There was another response, which he labeled the summating potential (SP) (Davis, Fernandez, McAuliffe, 1950). People began to realize that the CM and SP were interesting, but the largest component was a spike recorded in humans, which they called the action potential (AP).
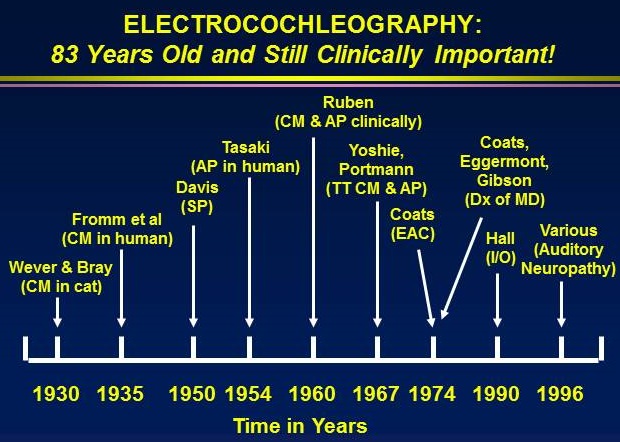
Figure 1. ECochG timelines.
By the late 1950s and early 1960s, audiologist Chuck Berlin and otolaryngologist Bob Ruben from Johns Hopkins said they could do this is in the operating room in children who could not be tested any other way. They used this as an electrophysiologic, objective hearing test. Unlike our small computers today, they had heavy racks of equipment that they would roll into the operating room. To get an electrode near the ear, they had to perform a myringotomy. For a child who definitely had a hearing loss, it was well worth it to make an accurate diagnosis of severity.
In the 1960s, people began to realize that recording the ECochG was not that difficult if they used a transtympanic needle, which is a sharp needle placed through the eardrum. It is barely invasive, and the eardrum heals right away. If you put it onto the promontory, you can still record these responses without going through the myringotomy process and making a slit in eardrum.
Al Coats was an otolaryngologist at Baylor College of Medicine back from the 1960s through the 1980s. He is credited for putting together the first electronystagmography (ENG) test battery. He was well-versed in vestibular studies, but he also did a fair amount of work with ECochG. He showed that these responses could be recorded less invasively from the ear canal. Coats, Eggermont, Gibson and others around the world began to realize that ECochG had value in the diagnosis of Meniere’s disease. Many of us who were getting into intraoperative monitoring the late 80s and early 90s started to adapt these procedures in the operating room with great success. Most recently, ECochG is being used in a lot of other ways, including the diagnosis of auditory neuropathy.
The ECochG has been fascinating. It became popular and then faded away. When ABR came along in the early 1970s, most audiologists forgot about ECochG. But now, I think it is back for good. It has too much to offer in the diagnosis of hearing loss.
The way the ECochG was recorded by the purists in the early days was to put an electrode on the eardrum or needle to the eardrum onto the promontory, or to do a myringotomy with a silver ball electrode. The silver ball electrode was a thin wire that was soldered and polished so it was smooth with a one- or two-millimeter silver ball. That would be placed right in the niche of the round window. When you place that non-inverting electrode and have the second electrode on the mastoid, for example, you end up with a waveform that is generated upside down. The N1, or the action potential (AP), rather than being a positive wave like we would record for ABR, is a negative wave. Large-amplitude responses are recorded deep within the ear canal on the bony part of the ear canal wall; promontory electrode recording produces responses of about 5 microvolts, which is much bigger. A recording from a tiptrode in the ear canal would be about 0.5 microvolts
The SP occurs just before the N1. We record the amplitudes of the SP and AP and mark the latency for each. Sometimes people will calculate the duration of each wave, particularly the SP and AP, because that can be abnormal in the case of Ménière's disease.
The three main components of the ECochG are the cochlear microphonic or CM, summating potential or SP, and action potential or AP. Every normal patient will have each of these three components. One of the features of ANSD is sometimes a very large CM. However, I have to point out that not all large CMs equal ANSD. The key is to have a CM but no ABR; that finding suggests ANSD. A large CM followed by an ABR is perfectly normal.
The CM is generated by the outer hair cells. When the stereocilia bend back and forth, they produce an electrical response at the apex of the hair cell, which is recorded as a CM. That is a normal occurrence. You cannot hear well without a CM. The SP arises primarily from the inner hair cells, although there may be other contributions from the rest of the cochlea including the stria vascularis. For the most part, the inner hair cells need to be intact to record a normal SP. That is a very important point when it comes to the diagnosis of ANSD. In summary, the SP is primarily produced by inner hair cell activity, the CM by outer hair cell activity, and the AP is exactly the same response as ABR wave I. It arises from the afferent fibers in the distal portion of VIIIth nerve nearest to the cochlea.
Within the organ of Corti, there is a row of inner hair cells and three or four rows of outer hair cells. The CM is generated from the very top of the outer hair cells. It does not have anything to do with the motility of the outer hair cells. The CM is recorded in a normal outer hair cell, and those patients would usually have normal OAEs. Occasionally, you will see a patient who has a CM but no OAEs. There are two explanations for that. The CM tells you that the hair cells seem to be functioning. The absence of OAEs might be due to a middle ear abnormality or it is conceivable there might be a problem with the up-and-down motility of the outer hair cell, but that is more of a metabolic abnormality; it does not involve the stereocilia.
Protocol
With the right electrodes, you can use virtually any ABR equipment to record ECochG. We typically use clicks to record the ECochG. Most people do not find any value in using tone bursts. We are not trying to estimate hearing; we are trying to evaluate the integrity of different components of the ear. Just as it is with ABR, we use a 0.1 millisecond duration, which is the same as 100 microseconds.
Let me just talk about the stimulus rate for a moment. If you are using a tiptrode in the ear canal, you are going to record a relatively small ECochG. Some people would say a tiptrode is not a proper ECochG electrode because it is too far away from the generator of these responses. If you do use a tiptrode, you need to use a much slower rate. The slower the rate, the bigger the response; the faster the rate, the smaller the response. If you are using a transtympanic needle that is positioned against the promontory, you are as close to the cochlea as possible from a peripheral location. With a transtympanic needle electrode, the rate of stimulation can be quite high and you will still record a large response. When I perform a transtympanic ECochG, I use a rate of 21 clicks per second or 21.1. If you use a tiptrode and do not get a large wave I, slow the rate down.
Polarity is another parameter that has to be manipulated when you are recording an ECochG. If you record an ECochG with alternating polarity, you will often get the best SP possible, a very nice AP, but no CM. If you use a single polarity, you are going to find that the CM direction will be opposite for rarefaction and condensation. Without using a single polarity stimuli, you will never record a CM because the alternating stimulus polarity will cancel it out. If you want to record ECochG correctly, you have to record multiple waveforms using rarefaction, condensation and alternating polarities. It would also be appropriate to record rarefaction and condensation individually and then add them together to make an alternating run.
A high stimulus intensity is required when we are recording a diagnostic ECochG. Some countries use the ECochG to estimate threshold if the ABR is not clear. They will use a transtympanic or myringotomy approach and use the responses to estimate hearing. It is very effective because the response is so big. Most American practicioners will use ABR or ASSR for that purpose, and then we will use ECochG to determine where the problem lies in the auditory system if there is a hearing loss.
Insert earphones are the best transducer for ECochG. You will never need to mask. This is an important point. If you have an electrode on the right ear lobe or right tympanic membrane and you stimulate the right ear resulting in a wave I, then you know that response is coming from the right ear. The contralateral ear could not be producing that response because the electrode that you are recording with is so close to the wave I generator. That is what I call a biological marker or an ear-specific response. The CM, SP and AP are coming from the patient’s ear and you are recording them with the electrode near that ear. If you wanted to prove that, do a two-channel recording and use your second inverting electrode for channel 2 on the contralateral ear, and you will see nothing. You may see a wave V further out, but you will not see any of the cochlear responses. You do not need masking for ECochG.
The left panel of Figure 2 is an example of a rarefaction ECochG from a patient with Meniere’s I saw years ago. You can see the up-and-down activity prior to the AP, which is the CM. Both panels show two separate, replicable waveforms. In the right panel, I switched to alternating polarity. You can see that the CM has disappeared, and we can identify the SP and AP very easily. The amplitude of the AP is 2.11 microvolts. This patient had a 55 dB sensory hearing loss throughout the low to high frequency regions. A response this large with hearing loss of that degree tells you that this response had to have been recorded using a transtympanic needle electrode. Notice the amplitude of the SP, which is 0.84 microvolts. We divide the SP by the AP to get the SP/AP ratio, which is 40% in this case. An SP/AP ratio of 40% in an ECochG using a transtympanic electrode is abnormally enlarged. In other words, the SP should be much smaller in a normal person.
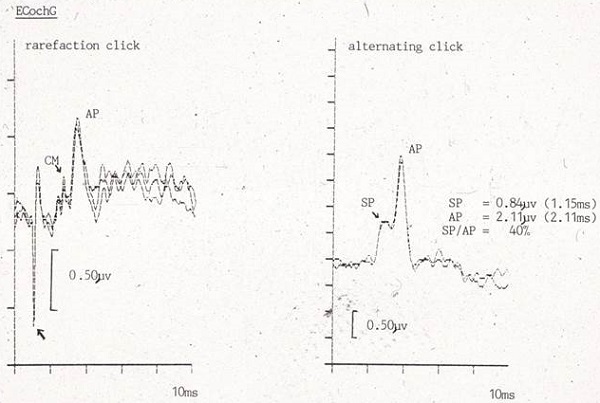
Figure 2. ECochG recording from a patient with Meniere’s disease; left panel= rarefaction stimuli; right panel= alternating stimuli.
Acquisition Parameters
The amplification needed for an ECochG is less than an ABR, particularly if you are using a transtympanic needle response. You can use a gain of 75,000 or less. Use an analysis time of 5 or 10 milliseconds. You want to highlight these early cochlear components. You can do a combined ABR/ECochG recording, but the danger of that is in using a very long analysis time, such as 20 milliseconds. In this case, you are cramming all of the cochlear activity in the first few milliseconds and it is very hard to distinguish the individual waves.
The National Hearing Service protocol used for diagnosing hearing loss in England requires 3,000 sweeps with a minimum of 1,500. However, because the electrode often is near the cochlea for an ECochG, the response is large enough that you do not need to average so much. I have recorded beautiful ECochGs using a transtympanic technique after 25 stimuli. Can you imagine if you are presenting 21.1 stimuli per second and you only need to average 25 to see a clear response? It takes it roughly one second to get the response. The key is the signal-to-noise ratio. If you are using a iptrode and the response is small, you do need to average more. I record at least four replicated waveforms of at least 500 sweeps each and then add them all together. Then you are really looking at a waveform of 2,000 stimuli.
You have to keep the low frequencies in the filter settings, because the SP is a direct current potential which contains a lot of low frequencies. I suggest using 10 to 1500 Hz. Additionally, a notch filter is never required. It will filter out too much of the ECochG activity.
Electrodes
If you want to get the best ECochG possible, you must use a transtympanic electrode. Any otolaryngologist can place a needle through the posterior inferior quadrant of the TM. You are not going to hit the ossicular chain. You are just going to go right back until you reach into the promontory with the needle, and then it will stay there. The tympanic membrane electrode is the best option for audiologists without access to an otolaryngologist. The least attractive and sometimes inadequate option is to use a tiptrode. In each of these cases, we have a non-inverting electrode on the forehead. The inverting electrode is in the ear canal.
I say that the tiptrode is the least attractive option because in patients with hearing loss, you might get such small responses that you can barely identify the AP, and there is usually no SP or CM. If your AP is less than 1 microvolt, by definition, your SP and CM are going to be tiny. I really want to emphasize this point. If you think a tiptrode is your only option, make sure you insert it as far down the ear canal as possible. It is physically impossible to get it on the eardrum because you are running into the bony part of the ear canal wall. If you want a clear, large ECochG, I do not recommend using tiptrodes, especially for patients with known hearing loss. You might be able to give it a try in children, but if it does not work, you need a backup plan. The very best option is a transtympanic needle electrode. The closer you can get the electrode to the cochlea, the better.
This all leads to a very important conclusion. ECochG is a near-field response. This means it is best recorded when you have an electrode within the electrical field-producing response. The ABR is a far-field response. The electrode is way up on the head, but the generators of the response are down in the brainstem, which we cannot access superficially. The amplitude is tremendously reduced in far-field responses. The response from the brain has to go through the dura, the skull, the skin and scalp and then to the electrode, so it is just a fraction of what it was to begin with. If you record an ECochG a millimeter from the generators, you will get responses that are 20 to 50 times bigger than ABR.
In 1974, Alfred Coats developed an electrode which was called the butterfly electrode or the Coats electrode. It was made from a very compressible, thin plastic. It was shaped like a V and was spring-like when squeezed together. It had a silver wire glued to it, and at the end of the silver wire was a small silver ball. To insert this electrode, compress the butterfly with bayonet forceps so it is close together, and then gently release it down in the ear canal. It keeps itself in place. This electrode allowed you to get very close to the bony wall of the ear canal. The concept was good. It was in the external auditory canal. It was noninvasive. It did take a little skill to place this and get it back out without lacerating the canal skin. It was very uncomfortable, however, because a lot of pressure was exerted by the silver ball on the delicate wall of the ear canal. I do know this from personal experience because I was a subject once when Al Coats was doing an ECochG workshop. I had blood coming out of my ear canal by the end of that session, which was not uncommon for this electrode.
Luckily, someone had the idea to put gold foil, which conducts electricity, on the outside of an insert earphone, and then have gold foil coming down the wire so that you could take an alligator clip and clip it on to the external foil (Figure 3). In the early years, as soon as insert earphones were developed, many of us took a silver wire and wrapped it right around the foam and connected that to an alligator clip at the other end of the insert. We made our own tiptrode ties, but now they are commercially available. I strongly recommend that you get a set of about 10 of these gold-foil inserts, one for each ear of five patients, and get a set of the special alligator clips. Any of the evoked response manufacturers will have them in their catalog. You might pay $50, but you can use the alligator clips over and over. That is a great asset. You are using the insert earphone to deliver the stimulus to the patient, but you are also detecting the response from the ear canal with the tiptrode.
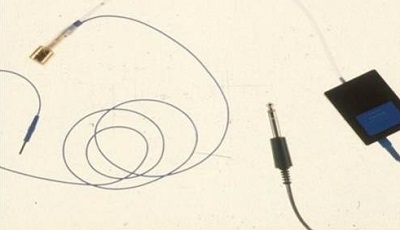
Figure 3. Tiptrode assembly using an EAR3 insert box.
The next step, which is ideal for audiologists, is to put an electrode on the eardrum. We used to make these by taking a silver wire and threading it through some probe microphone tubing. We put a small piece of gauze or cotton at the end. We would soak the cotton in saline, slip it down into the ear canal to the ear drum and have an alligator clip that would allow you to get the regular silver wire to your electrode box. We then put the insert earphone in the canal to hold the wire in place. Biologic made these tympanic membrane electrodes for a while, but have since discontinued the product. Intelligent Hearing Systems now makes them. They are FDA-approved. The silver wire has an electrode pin and plugs into a regular electrode box.
The best option is the transtympanic needle electrode (Figure 4). This is done in the operating room. The needle is very short at about 14 mm. The tip of the needle goes on the promontory. When you get this in place, you place an insert earphone into the ear. You can tape the wires to the cheek to make sure they do not get pulled (Figure 5). There is no way the needle is going to come out unless you pull it out. Some worry about putting a hole in the eardrum, but if you were to do a tympanogram the next morning, the eardrum would be healed.

Figure 4. Subdermal needle electrode.
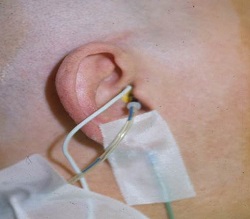
Figure 5. Subdermal needle electrode placement in the ear canal with insert earphone in the ear canal.
Figure 6 shows a comparison of an ABR recorded in the operating room using a tiptrode versus a needle electrode. The recordings at the top were taken using a tiptrode in the ear canal; they show high amounts of background noise from the various equipment used in the operating room. You can identify wave V if you know what you are looking for, but it is not very clear. By the end of the case (Figure 6, right panel) there was so much artifact from additional equipment that you can barely see a wave I or wave V. Compare this to a forehead-to-promontory two-channel recording done at the same time. Waves I through V are all identifiable and there is virtually no artifact. The size of the response is decent also; 10 to 20 microvolts is very typical.
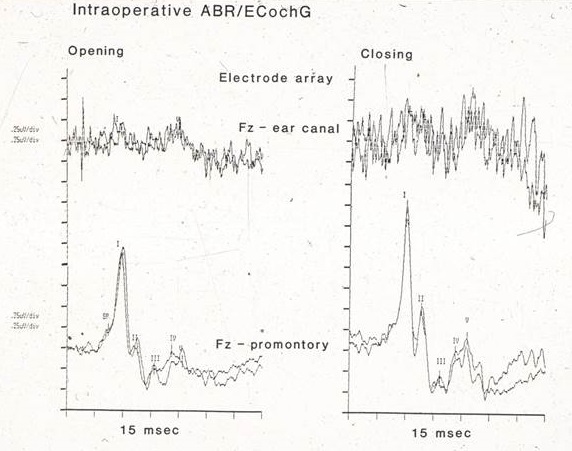
Figure 6. Intraoperative ABR/ECochG comparison using tiptrodes (top recordings) versus needle electrodes (bottom recordings).
Clinical Applications of ECochG in Adults
The one application of ECochG used most often in adults is the enhancement of wave I in the ABR. To do this, you can slow down the rate or try a different polarity. That is very important. The bigger the wave I, the more accurately you can calculate inter-wave latencies. You can make sure the response is truly coming from the test ear. I have already mentioned that intraoperative monitoring is greatly enhanced by the use of ECochG, as is the diagnosis of Meniere’s disease. That was first introduced by Coats (1981) and others in the early 1980s, and it is still used by some audiologists who are experts in Meniere’s disease.
Al Coats (1981) and other early researchers realized the SP and the AP for different patients varies tremendously. Using an absolute amplitude for the SP to define normal versus abnormal does not work. However, when you compare the patient's own SP amplitude to their AP amplitude, you are normalizing the data and you get a well-defined normal range. The SP/AP ratio of retrochcochlear patients falls well within the normal range; the majority of patients with cochlear hearing loss also falls within the normal range. Patients with Meniere’s disease have a different pattern. Many have large SP amplitudes when compared to AP amplitudes. That is characteristic of Meniere’s disease. You can find more information in the literature by doing a Medline search using the keywords Meniere’s disease and electrocochleography. I came up with 1,400 publications.
Clinical Applications of ECochG in the Diagnosis of ANSD
ECochG can help sort out where the dysfunction lies within the auditory system. If you can determine where the dysfunction is in the auditory system for a patient suspected of ANSD, then that is the most important finding leading to proper management of that patient. Is the problem in the cochlea, which technically, then, is not a neuropathy? Is it the synapse at the base of the inner hair cells, or is it the auditory nerve? Any disorder that affects the central nervous system but leaves the peripheral part of the auditory system intact is not auditory neuropathy by our definition.
I would suggest you download the document Identification and Management of Infants and Young Children with Auditory Neuropathy Spectrum Disorder (2008) from the Bill Daniels Center for Children’s Hearing in Colorado (https://www.childrenscolorado.org/pdf/Guidelines for Auditory Neuropathy - BDCCH.pdf). These are the definitive guidelines for identification and management of auditory neuropathy. A panel of experts, with the guidance and inspiration of Deborah Hayes, put this document together. If you want to know how to identify, diagnose, and manage auditory neuropathy following evidence-based best practices, these are the guidelines you should use.
According to these guidelines, the minimum test battery includes tests for cochlear hair cell function, the CM and auditory nerve function. Additional tests may include acoustic reflexes and OAE suppression. The ECochG fits the bill to test for hair cell function and the CM.
Figure 7 shows recordings from a patient when I was at Vanderbilt. This is not the diagnosis of auditory neuropathy; this just raises the suspicion. This patient is a twin, and one of the twins failed the ABR screening in the intensive care nursery and the other child passed. When they came back at three months for follow-up, this is what I got with one of the twins (Figure 7). The other baby had a normal ABR. At 95 dBnHL, the waveform that you see is all CM. I changed the polarity of the stimulus, which completely changed the phase of the response. When I added the rarefaction and condensation together, it resulted in flat line. Every time there is a peak in one place, there is a valley in the other. There was no ABR, only CM. That is one reason why we do not want to use alternating polarity routinely to complete the ABR. If you do OAEs in patients like this, they will typically be normal, and sometimes even a little bigger than normal. A typical pattern is to fail the ABR screening but passing an OAE screen. It is not a diagnosis, but it should make you suspicious.
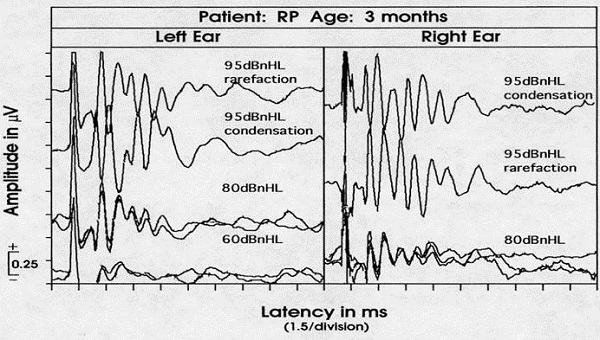
Figure 7. ABR recording from a 3-month old infant who failed a newborn hearing screening; rarefaction and condensation responses are recorded and appear out-of-phase.
If you follow these patients, you will almost always find that they have a concurrent disease or diagnosis. There is a certain category of patient who only has an auditory problem, but many of these patients have neurologic disorders, such as hyperbilirubinemia, ischemic or hypoxic insults to the brain, hydrocephalus, immune disorders or demyelinating diseases. Over time that diagnosis becomes clear, but through your ECochG, ABR and OAEs, you may be the first person to suggest to a physician that perhaps there is a neurologic problem. Up to half of all patients with a diagnosis of ANSD have a genetic or hereditary etiology. A referral to a geneticist is mandatory in patients who are diagnosed with ANSD. Some of these patients will end up with very serious neurological disorders that are degenerative. Others will end up with cochlear problems that are not neural and are genetically determined.
Beyond the auditory tests, there are other diagnostic tests that must be used. Imaging of the auditory nerve with MRI is critical. Every patient worked up for auditory neuropathy must have an MRI of the auditory nerve. Here is why. Research clearly shows that one out of five patients (20%) with ANSD actually has cochlear nerve deficiency and sometimes no nerve at all (Huang, Roche, Buchman, & Castillo, 2010; Liu, Bu, Wu, & Xing, 2012). If the ABR is absent but you record normal OAEs and a normal CM, it is very important for the patient to get an MRI of the auditory nerve to rule out cochlear nerve deficiency. If a patient has no auditory nerve, a cochlear implant is not an option. Interestingly, when patients have bilateral cochlear nerve deficiency, they are very likely to have intracranial problems as well. Your finding of ANSD can sometimes lead to valuable neurologic information on the child.
We can differentiate these three sites of dysfunction (outer hair cells, inner hair cells and auditory nerve) by using ECochG, and that will lead to proper management. Recent research supports this claim (Santarelli, Starr, Michalewski, and Arlsan, 2008). One study is from well-known auditory physiologists in Australia (McMahon, Patuzzi, Gibson, & Sanli, 2008). They used a group of bilateral ANSD patients who received cochlear implants. Half of them were not doing well with the implants and the other half were doing very well. Retrospectively, they went back and did ECochG on the non-implanted ear using an electrode placed in the round window area. Six out of 14 subjects had a genetic etiology for their ANSD.
The authors (McMahon, Patuzzi, Gibson, & Sanli, 2008) found that patients who had normal inner hair cells and a normal SP, but no AP, did very poorly with the cochlear implant. The nerve fibers were not able to be stimulated or were not functioning. On the other hand, patients with the presynaptic ECochG pattern where the SP was missing, indicating inner hair cells were not functioning, did have CMs, but none of them had ABR, including wave I. Those who had an abnormal SP did very well with the cochlear implant. The cochlear implant was essentially substituting for the abnormal inner hair cells. Those patients’ MRIs all showed an auditory nerve, so the cochlear implant was able to stimulate the nerve.
The management of ANSD is beyond the scope of this lecture, but let me say that monitoring this diagnosis with behavioral findings is very important until you finally determine what the behavioral hearing status is. Monitoring with OAEs is important, as is referral to other disciplines including genetics, ophthalmology and ENT. Try hearing aids. Roughly half of all patients with ANSD do well with hearing aids. That has been proven over and over. Hearing aids can be appropriate for this population, often with the use of an FM system. If the problem is presynaptic, cochlear implants are a great option and those patients usually do well, particularly when their central nervous systems are normal. Of course, if the nerve is not functioning at all and if a cochlear implant is not an option, then we have to look at alternative strategies.
Summary
The ECochG still has value in clinical audiology, both for adults and children. We all can apply ECochG principles and techniques in recording ABRs, or we can record a pure ECochG using a transtympanic or tympanic membrane electrode. You can modify the protocol to enhance the CM, SP and AP as much as possible.
If you want to download other lectures I have given, you will find them on my Web site at audiologyworld.net along with other useful information.
Questions and Answers
What is the relevance of the SP/AP ratio?
If you are using ECochG to enhance wave I of the ABR or you are using ECochG in a patient to help you diagnose auditory neuropathy, you are not too worried about the SP/AP ratio. On the other hand, let’s say you have a patient with vertigo that suggests Ménière's disease, but there is no firm diagnosis. If you have a large SP/AP ratio that exceeds normal limits for the electrodes you are using, that is strong evidence of Ménière's disease.
Let's say you have a patient where there is clearly Ménière's disease in one ear, but the question is the other ear. The management of the patient may not be very successful if only one ear is treated, because the other ear will still cause problems and create vertigo. In this case, you can use ECochG on both ears. If you get the same abnormal pattern in both ears, you will know that the second apparently-normal ear is certainly not normal. The diagnosis is likely early Ménière's disease on that side, too.
How do you properly insert the tiptrode?
The tiptrode is an insert earphone covered with gold foil. You take a Q-tip and put a little abrasive liquid on the end. Pull the ear up and back to straighten the ear canal. Gently scrub the outer third of the external ear canal, and slide the tiptrode in. Do not put any gel on the tiptrode. You want as deep of an insertion as possible to get the tiptrode closer to the cochlea.
How would the post-synaptic pattern in ECochG in cases of ANSD affect the decision of cochlear implantation?
If there is a post-synaptic pattern suggesting or providing some evidence of an auditory nerve abnormality, you need to proceed very cautiously. I do not think anyone would jump to the conclusion that a cochlear implant would help that patient. The first thing that would need to be done is an MRI. If the MRI showed an intact nerve, another option would be to say that we know there is a postsynaptic problem, but we do not if those nerves are excitable. They show up on the MRI, which is a good thing, but let's do an electrical mid-latency ABR or an ABR in young children because then they can be sedated to do it.
You could use the same transtympanic electrode that you used to record the ECochG to present the electrical stimulation to find out if the nerve is intact. There are otolaryngologists who have said, “I know that the inner hair cells are intact, but the patient is not developing speech and language, so it is a postsynaptic problem. It is not the inner hair cells, it is the nerve.” They think the nerve is going to be able to handle stimulation. They have proceeded with cochlear implantation, and some of the patients have done well with cochlear implants; others have not. I should point out that an ASSR can be useful. The ASSR uses a pure-tone stimulus. It is not an abrupt, transient stimulus. If you have a patient who has any ASSR activity, even if the ABR is absent, that usually means the auditory nerve is capable of firing. It is getting information to the brain, and often it means that the abrupt click or tone burst stimuli for ABR are not very efficient in activating the nerve. But it shows that the nerve can function, and that is a good sign for cochlear implantation.
References
Bill Daniels Center for Children’s Hearing in Colorado. (2008, June). Guidelines for identification and management of infants and young children with auditory neuropathy spectrum disorder. Retrieved from https://www.childrenscolorado.org/pdf/Guidelines for Auditory Neuropathy - BDCCH.pdf
Coats, A. C. (1981). The summating potential and Meniere's disease. Archives of Otolaryngology, 104,199-208.
Davis, H., Fernandez, C., & McAuliffe, D. R. (1950). The excitatory process in the cochlea. Proceedings of the National Academy of Sciences of the United States of America, 36(10), 580-587.
Huang, B. Y., Roche, J. P., Buchman, C. A., & Castillo, M. (2010). Brainstem and inner ear abnormalities in children with auditory neuropathy spectrum disorder and cochlear nerve deficiency. American Journal of Neuroradiology, 31(10), 1972-1979. doi: 10.3174/ajnr.A2178
Liu, C., Bu, X., Wu, F., & Xing, G. (2012). Unilateral auditory neuropathy caused by cochlear nerve deficiency. International Journal of Otolaryngology, 2012, 914986. doi: 10.1155/2012/914986
Wever, E. G., & Bray, C. W. (1930a). Action currents in the auditory nerve in response to acoustic stimulation. Proceedings of the National Academy of Sciences of the United States of America, 16(5), 344-350.
Wever, E. G., & Bray, C. W. (1930b). Auditory nerve impulses. Science, 71, 215. doi: 10.1126/science.71.1834.215
Cite this content as:
Hall, J.W. (2013, October). Clinical applications of electrocochleography in audiology today. AudiologyOnline, Article 12095. Retrieved from: https://www.audiologyonline.com


