Interview with Steve Hawkins and Pamela Matthews Audiologists with SOUNDTEC Inc., Manufacturer of Middle Ear Implants
AO/Beck: Hi Steve and Pam. Thanks for joining me today. I have read a little about the middle ear implant, and I'd like to explore that this morning.
Hawkins: Hi Doug, great to be with you.
AO/Beck: I wonder if you would each spend a few minutes telling me a little about your professional history, before we get into the new device?
Hawkins: Absolutely. I got my master's degree from the University of Missouri at Columbia in the late 1970s. I ran a few clinical dispensing offices in Central Texas until the mid 1980s. From 1986 to 1993, I worked in the northeast for Cochlear Corp as a Regional Manager and then from 1993 until 1999 I worked for Advanced Bionics in North America and the Pacific Rim, establishing new centers for the Clarion cochlear implant. For the last year, I've been the Vice President of Sales and Marketing for SOUNDTEC. The company was founded about 4 years ago by a small team which included Dr. Jack Hough. Doug, as you know, Dr. Hough is an otologist and he has pioneered other middle ear implant systems going back to the 1980s. Additionally, he and his team developed the magnetic interface which attaches the internal and the external components used by all of the cochlear implant systems.
Matthews: I've been with the Hough Ear Institute for 13 years as a research and clinical audiologist. As you know, Dr. Hough is one of the founders of SOUNDTEC, and as the company grew, I became more involved. I am the Director of Clinical Studies for SOUNDTEC. I also train other audiologists and work with patients on the clinical selection, testing and 'tune-up' aspects.
AO/Beck: Steve, tell me about the new device please.
Hawkins: The SOUNDTEC middle ear implant is called 'The Direct System.' It is a very small, and very simple device. The implantable part is a hermetically sealed rare earth magnet, encased in titanium. It is attached to the head of the stapes at just about the incudo-stapedial joint and is held in place with a tiny ring. The size of the implant is about equivalent to one third the size of a grain of rice. The surgery takes about 30 minutes under local anesthesia. The external device is currently housed in a BTE type device with a deep 'ear mold.' The BTE unit is the processor, and the earmold section transmits the electro-magnetic signal to the implanted device. Importantly, the earmold doesn't have to fit tight -- as it would on a standard device. Again, because the signal leaving the earmold is electromagnetic, there is no acoustic feedback. So although we like the earmold to be as deep as possible, it's very comfortable because it doesn't have to hug the canal wall, and in fact it can be a loose, non-occluding fit, because there is no acoustic signal present, and therefore no feedback. See the illustration below for a little better idea of this technology.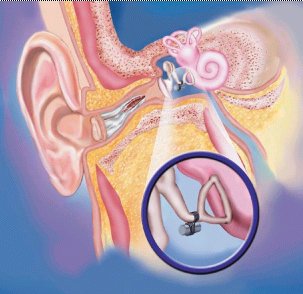
Matthews: As Steve mentioned, the current approved device is a BTE device, and that is the one the FDA has approved. However, over the next few months we hope to release the ITC/CIC versions, and these will be available as upgrades to the patients at no additional charge. Of course the exact timetable for the in-the-ear models is unknown, but we'll do it as quickly as possible because we know that cosmetic issues are not trivial.
AO/Beck: Importantly, the earmold cannot be made in a regular earmold lab, right?
Matthews: Right, the ear impression is sent to SOUNDTEC, and we create the special electromagnetic earmold.
AO/Beck: How many SOUNDTEC implants have been done worldwide?
Hawkins: As of this moment, 103 have been implanted through the clinical studies in the USA. We started implanting these in 1998 to meet the FDA's required protocols.
AO/Beck: Who is the ideal candidate for this device?
Matthews: There is a large range. Essentially, the patients are all adults with sensorineural hearing loss. The hearing loss ranges from mild sloping high frequency loss, to moderately severe flat losses.
AO/Beck: What is the period of time between the surgery and the initial 'tune-up?'
Matthews: We usually wait about ten weeks, although I think some physicians may cut that down a little, perhaps 8 weeks or so depending on healing time and related factors.
AO/Beck: Tell me about the technology, it this version digital or analog?
Hawkins: The current device is analog and it's a 'stand alone' version of a two channel, wide dynamic range Gennum processor. The digital product will probably be out in a year. The battery currently lasts about 2 to 4 weeks.
AO/Beck: What is the retail price the patient can expect to pay for the device and the surgery combined?
Hawkins: That's a great question, and - I'm sure that's on everyone's mind. The cost per ear runs between 4000 and 6000 dollars for a monaural fit, including the device and the surgery. Most of our patients have been fit monaurally, but obviously the binaural advantages are being investigated and we anticipate that will be the direction most of these goes in the near future.
AO/Beck: How has the SOUNDTEC performed in your studies when comparing the SOUNDTEC to DSP hearing aids?
Matthews: When comparing monaural SOUNDTEC to monaural digitally programmable hearing aids, using the HINT test, the patients with the SOUNDTEC did as well as the patients with the digitally programmable hearing aids but, as mentioned before, did not experience feedback, distortion or the occlusion effect.
AO/Beck: What is the fitting protocol you recommend?
Matthews: Because we don't really run into feedback issues, we basically target about half the loss as the target gain. So for a 50 dB loss you can target about 25 dB of gain.
AO/Beck: Please tell me the location of the website for folks looking to get more information on the SOUNDTEC middle ear implant?
Hawkins: The website is at www.soundtecinc.com, and the toll free phone number is 1-800-793-9587.
AO/Beck: Very good. I think the information above should help get everyone up to speed on these issues and help everyone figure out what you've got. I would really like to get some clinical data as soon as possible, and if you can provide me with that, we can get an article out via Audiology Online, hopefully over the next month.
Matthews: Sure Doug, we'll do our best to get that to you very quickly.
AO/Beck: OK, thank you both for your time this morning and we'll look forward to learning more about the product over the next few weeks.
Hawkins: Thank you Doug. We appreciate the opportunity and we're happy to work with you and Audiology Online.
