Editor's note: This is a transcript of an Advanced Bionics' live webinar on AudiologyOnline. Please download supplemental course materials.
Welcome everyone. I would like to thank Advanced Bionics for inviting me to present today. I am pleased to see that there are so many people interested in this topic. It has certainly consumed a lot of my time and thought in the last few years, and I think that there is still so much for us to learn. I feel like there are new things revealed to us on a weekly basis, especially with some of these very complex patients.
I am at the University of North Carolina (UNC) which is in Chapel Hill, and the center is a large tertiary care center. Most of our children come from within the state, but we also see a few from surrounding states. We have a large pediatric audiology program and see a lot of children who are newly identified, over 1,000 that are using amplification, and over 800 with cochlear implants. Among these patients, at least 200 have been diagnosed with auditory neuropathy spectrum disorder (ANSD). This has become easier since universal newborn hearing screening came into play in North Carolina in 1999. I have been with UNC since 2003. I am very lucky to have a dedicated and distinguished group of colleagues to work with. We have a large audiology faculty at the hospital, and they do a lot of the early identification diagnostic care and hearing aid fittings. When a child seems to be a candidate or is appropriate to start considering cochlear implantation, they make the referral over to our pediatric cochlear implant program, which houses another set of audiologists. We have a great staff of speech pathologists who work with us there. We do in-house therapy. We have a preschool program and have many parent sessions. We definitely believe in a strong team approach. We have four excellent surgeons and two wonderful auditory physiologists that work with us. I would like to acknowledge them and thank them for their hard work.
I think this topic is one that has called for a lot of conversation and investigation. If you do a literature search now, there is more and more on this topic all the time. It is one of those things in audiology that is so exciting because there is always something new. I know when I first relocated here, it was not very much on my radar that I would be seeing a lot of children with ANSD, and it really has been a big part of our thought process and patient management with how to take care of these very diverse patients.
As a means of an outline, I wanted to start with an overview and definitions, some of the variations in presentation that we have seen and that others have reported, the electrophysiological findings that are becoming more and more helpful in making the diagnosis and managing the children, some of the clinical outcomes that we have seen, as well as implications for how we use this information to manage our patients.
Overview and Definitions
As most of you know, auditory neuropathy is a clinical syndrome that is characterized by the electrophysiological evidence of near-normal or normal cochlear function and abnormal auditory pathway. Very typically, tests show us that there is normal outer hair cell function as measured by the presence of otoacoustic emissions (OAEs) or a cochlear microphonic, but there is abnormal auditory nerve response with a markedly abnormal or absent auditory brainstem response (ABR), and acoustic reflexes are typically absent. Other reported clinical characteristics of ANSD include pure-tone hearing thresholds that range anywhere from normal hearing to profound loss. It has often been noted that there is a disproportionately poor speech recognition ability considering what the pure-tone hearing levels are. These patients may experience increased difficulty in noise, impaired temporal processing, and often hearing fluctuation. All of this plays out differently in different individuals. Some people have ANSD and have no idea that they have it, other than some noted difficulty in noise or trouble in some environments at some time in their life. Others are highly impaired by it. Everyone seems to rely on the phenotype, what we see, and many people have reported on these different examples.
Initially, ANSD was not called ANSD. It has had a number of different names, but there was a consensus meeting in 2008 where the term Auditory Neuropathy Spectrum Disorder (ANSD) was coined. It seems to be acceptable to most people in that it hits all aspects of it. Initially, the disorder was not well known and was thought to be rather rare. In the late 1990s, we began hearing more and more about it. Gary Rance (2005), who has done a lot of the seminal research in this area, at one time estimated that 7% to 10% of children have ANSD, but I would suspect now that more people are learning how to identify it, that number might be slightly higher.
The criteria to diagnose ANSD from an audiological standpoint requires a test of cochlear hair cell function, which would be OAEs or cochlear microphonics, and then also a test of auditory nerve function, which is the auditory brainstem response (ABR). Some of the references have very well-explained descriptions of how the ABR should be conducted in order to gather this information and make the diagnosis of ANSD.
At UNC when we identify a case of ANSD, we also follow with some other diagnostic evaluations. These include a complete otologic evaluation, imaging, likely a neurologic eval if that seems warranted, medical genetics, which some may or may not choose to follow through with, an eye exam, and then pediatric and developmental evaluations as needed. We do a speech and language assessment on all the children as well. The reason it is so important to do a thorough medical evaluation is because there is such as high percentage of children with ANSD who have co-morbid conditions, and, in fact, ANSD is probably a result of some of these conditions. In a review paper that we did looking at medical diagnoses that go along with ANSD, there were only about 28% that really had no other medical diagnoses, but many of these other diagnoses are ones that we find from children who have spent some time in the NICU or who were premature (Ref, XXXX).
There have been a number of clinicians and researchers who have described different etiologies and associations, and certainly there are a few case studies out there that speak to some of these (Rance, 2005; Rapin & Gravel, 2003; Starr, Michalewski, Zeng, Fujikawa-Brooks, Linthicum, et al., 2003; Hayes, 2011). The non-syndromic etiology of the Otoferlin gene is probably the most written about. Oftentimes those children have no other co-morbid conditions. As I mentioned, perinatal conditions seem to be the cause for much of our population’s ANSD, in our clinic anyway. Some of these conditions include hyperbilirubinemia or kernicterus, low birth weight, oxygen debt, and struggles associated with premature infants. Other etiologies that have been known to be associated with ANSD include cochlear nerve deficiency, infectious processes such as mumps and meningitis, and head trauma such as shaken baby syndrome (Rance, 2005; Rapin & Gravel, 2003; Starr et al., 2003; Hayes (2011); Buchman, Rousch, Teagle, Brown, Zdanski, et al., 2006).
There are many challenges and questions that I hope to talk about and address today. Making the diagnosis is well-documented, and I will review that quickly, but I think counseling families is a very critical part of what we do as audiologists. One of the least helpful things that we can do when we are counseling families is add to the mystery of this diagnosis. We should try to help parents understand what it is, what we do know about it, and use evidence-based research and information to help them understand and to make their options for treatment clear.
A big challenge for all of us is determining if hearing aids are going to be helpful and if they are going to be a long-term or short-term solution. How long do we try hearing aids on a child with ANSD if they are muddling along and making some progress? There are no easy answers to some of these questions. How do we determine who is going to benefit from a cochlear implant? Are there alternative cochlear implant speech-coding strategies or techniques that might result in better performance? I am going to share a little bit of our experience with that. Lastly, what communication approach is best? You can imagine that is a very individualized discussion that has to occur with each family.
Diagnosis of ANSD
There are many audiological tools we use to make the diagnosis. We absolutely need an ABR. We always do tympanometry, acoustic reflex testing, and OAE testing. Those are the objective tests that can tell us whether or not there is outer hair cell function and some problem with the auditory nerve. The gold standard, of course, is behavioral audiometry. We work very hard to get visual reinforcement (VRA) and conditioned play audiometry (CPA) results in place so that we can do an appropriate hearing aid fitting as soon as possible. Certainly, however, when we cannot rely on the ABR to give us estimates of hearing thresholds for the hearing aid fitting, it does delay the fitting of the hearing aid until we can get some behavioral information. Speech recognition testing is critical as children mature and become capable of participating when we are trying to make a decision about whether a cochlear implant might be a better option for them. There are many reasons to do speech recognition testing that are beyond the scope of this presentation. However, it is an important part of our pediatric follow-up.
Figure 1 is often what we see with on an ABR in a child as we diagnose ANSD. You will see a lack of later reproducible waves, but a frequency-following cochlear microphonic, which can be very large or small. You can often see a robust response from outer hair cell function on the OAEs. This is fairly typical.
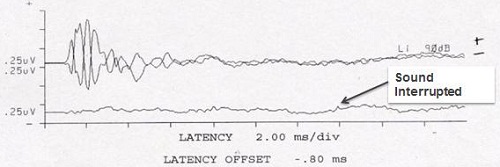
Figure 1. ABR tracings in the presence of ANSD (top), with present OAEs (bottom).
Just a word about the cochlear microphonic: it is a pre-neural response and shows a direct phase relationship to the acoustic wave form. It is important not to use alternating polarity as your only run, because it will cancel out the response. Rather, use both condensation and rarefaction runs. In the past, the cochlear microphonic has not been considered to be very useful, but it is a primary tool now in the diagnosis of ANSD. A present cochlear microphonic and no later wave forms is our marker of a positive diagnostic test for ANSD.
Our protocol at UNC for children is to do the electrophysiologic testing and then begin behavioral assessments with VRA as soon as the child is able. Six or seven months is typically the age where it is possible to start doing testing in the booth and getting reliable behavioral responses. We try to get conditioned responses. Behavioral observation is sometimes all that is possible, but we really work hard to get the conditioned response. It is important to remember that many of these children have additional developmental delays, so they may not be able to sit up, turn their head or participate in testing. Sometimes the process can be a bit delayed when getting behavioral information, and sometimes we never get good behavioral information depending upon the child and other medical issues.
We fit amplification as soon as we get ear-specific information using standard Desired Sensation Level (DSL) protocols and fit to optimize audibility. The hearing aid fitting alone, of course, is not the end, and there are a lot of things that have to be monitored and will impact the child’s success with the hearing aid. As all clinicians know, the age at diagnosis, treatment, consistency in using the device, quality of early intervention, involvement of the family, and the child’s other diagnoses or developmental delays all play into the overall picture.
Gary Rance and colleagues (2002) wrote one of the seminal research articles looking at the benefits of hearing aids for children who have been diagnosed with ANSD. He has published more findings since then, but in the original article, he found that of the children he evaluated with hearing aids, about half of them showed significant open-set speech improvements and the other half did not. Certainly you do not know which half will and which have will not until you do an appropriate hearing aid fitting. In his recent work, he has shown that there are some children with ANSD who do better with hearings aids (Rance & Barker, 2008; 2009). He did a comparison of children with hearing aids, children with implants who had ANSD and then children with implants who had sensorineural hearing loss. He generally found that the children with ANSD were not performing as well. Other researchers have found some slightly different results, in that there is not as much difference between children with ANSD and sensorineural hearing loss. I am going to touch on that a little bit later.
ANSD and Cochlear Implants
The questions I want to answer today are, “What have we learned about ANSD and cochlear implants? What are we continuing to learn? How does this impact implant candidacy and counseling? How do we manage those children?”
Most of you work with or have some exposure to cochlear implants, I assume. I would remind everyone that the criteria for cochlear implantation for children has not changed since 2000. Advanced Bionics does not have a hearing level criterion, but Cochlear Corporation and Med El specify in their package inserts that hearing levels in the severe to profound range are what are required. As many know, we cannot only look at hearing levels when it comes to implant candidacy. We need to look at auditory development, speech recognition performance and a child’s progress over time. Insurance companies may still be looking at these very dated criteria for implantation, but I think in clinical practice we know that cochlear implants provide better results than hearing aids for children who are well outside this criteria.
What have we learned?
I am going to tell you the answer to this question first, and then go back and tell you why I think we have learned that. We have learned that etiology does matter. There is tremendous variability among children who have ANSD, and these co-morbid conditions affect performance and make patient management very challenging.
Let me tell you how we have learned that. In 2006, there was a study (Buchman, Roush, Teagle, Brown, Zdanski, & Grose, 2006) looking at the presence of cochlear malformations and, specifically, what was coined cochlear nerve deficiency. That may not mean a complete absence of the nerve, but it could mean a compromised nerve as shown by MRI. Of the 140 children that we had available MRI data on, 25% of them with ANSD had some degree of cochlear nerve deficiency. It was not always bilateral. In many cases it was unilateral, and in some cases, the opposite ear was a normal hearing ear. These results were published, and certainly the discovery of the importance of MRI to the diagnostic process and the counseling process cannot be underestimated. Cochlear nerve deficiency is not really uncommon. You may have a CT scan that looks perfectly normal, but when you do the MRI, you can see that there is an absence of the auditory nerve in the internal auditory canal. The surgeon and radiologist team at UNC have developed a protocol to do this imaging, and it has been very helpful in knowing this information when a child is diagnosed with hearing loss so that we know what treatment options make sense.
We did a retrospective review of all the patients who had been identified with ANSD or followed at UNC and published the findings (Teagle, Roush, Woodard, Hatch, Buss, et al., 2010). We had 140 patients: some of them using cochlear implants, some using hearing aids, and some using no assistive device at all. They were all evaluated by a pediatric audiologist and an otologist. Thirty-seven percent of our patient base (58 total) ended up receiving cochlear implants. Fifty had bilateral ANSD and eight had unilateral ANSD. Fifty-two went on to get cochlear implants in the ANSD ear. I think this speaks to some of the Rance (2002) data indicating that some children actually are benefitting from the use of hearing aids and need to continue to use hearing aids.
Twenty-eight percent of the entire group had no assistive device, and that may be because, at the time, we were in the process of gaining information about their behavioral hearing thresholds, or it may have been that amplification was not recommended for them. Six percent of the children used a cochlear implant and a hearing aid together. I am going to mention here again that this group has significant other medical conditions. A high percent of them had conditions that we see in NICU babies; only 22% of them had no other medical diagnoses. Some had the Otoferlin type of diagnosis, but for the most part, these are children who are not easy to manage in the clinic because of their multiple needs.
Identifying these candidates for implantation takes a little bit longer if they have more residual hearing. We did not always have the option of using hearing preservation electrodes, so when a cochlear implant was placed, that meant loss of residual hearing. Some children were left with what we call bathtub hearing. This is when you take the cochlear implant off and still have a little bit of sound awareness. Children with ANSD who had unaided hearing thresholds and got a cochlear implant often lost that unaided hearing. Generally, we concluded in our study (Teagle, et al., 2010) that the more hearing the patient had, the longer it took for us to recommend a cochlear implant for them. It was part of the watch-and-wait strategy where we followed carefully to see if they were making progress and if that progress was adequate.
Of the 52 children included in the publication, there were about 29% who had no open-set speech recognition ability and some that were very difficult to test. There were 35% of the children who actually achieved significant open-set word performance and 15% who scored less than 30% correct on open-set word recognition, which, of course, is the general candidacy criteria for cochlear implantation in children.
Figure 2 shows word recognition scores on the PBK for various subjects based on performance. Several children, even after getting their cochlear implants, were scoring poorer than implant criteria, but then we had some very impressive results with children scoring very highly on word recognition, also.
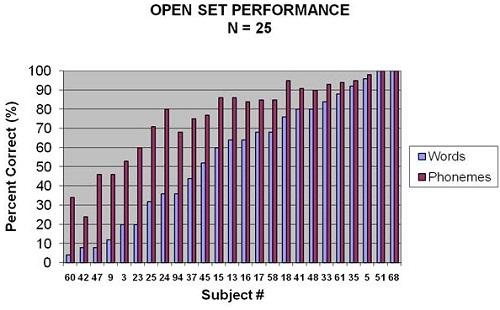
Figure 2. Word recognition scores in percent correct for 25 of 52 subjects of the study. Results shown are for words (light blue) and phonemes (purple).
Interestingly, when we look at these same data based on duration of use (Figure 3), you can see that these varied scores are not a result of the amount of time the patients had used their cochlear implant at all. We have children with a very short duration of use who do very well, and we have children who have used their device for years and still are not making good progress. Length of use was not really a factor there.
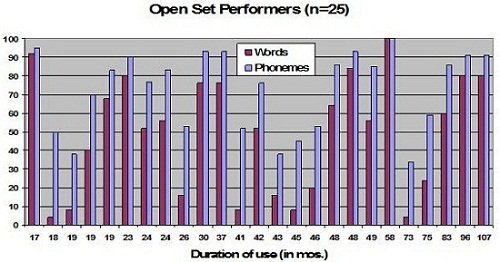
Figure 3. Word recognition scores for the same 25 subjects as a function of duration use in months.
It was interesting, as well as a little discouraging, to learn that only about half of the children were making significant gains, but some of those children simply were not testable. Because of their other medical conditions, we did not have the data to show speech recognition ability. You really cannot discount how much sound awareness adds to quality of life.
We used other measures such as the Infant-Toddler Meaningful Auditory Integration Scale (IT-MAIS) or parent questionnaires to gain information about how beneficial implants are to this population. All of the children that were in the study were full-time users of their cochlear implant, and the fact that we did not have open-set speech recognition scores on some of them may be a result of their other medical conditions and their inability to participate in testing.
We took a second look this year at this group of children. We ruled out all of those who had multiple medical diagnoses and did a match between a child who had a cochlear implant and a child with sensorineural hearing loss. We matched them on a number of factors to see how they compared. Of our over 800 children who have received cochlear implants, there were 90 who had the ANSD diagnosis that we knew of, because we were not making the routine diagnosis before 2000. We compared the sensorineural hearing loss children to the ANSD children and looked at their speech-perception abilities. The way we compared them was by using a formula that has been used in the CDaCI, Communicative Disorders after Cochlear Implant study (Wang, Eisenberg, Johnson, Fink, Tobey, et al, 2008) which is a multi-center study that used a cumulative quotient for expressing speech perception ability. Basically, it is a hierarchy of tests that you add up, each test having 100 points. If a child got 100% on the IT-MAIS, they started at 100. If they were able to do some closed-set measures after that and scored 100%, they were at 200. The hierarchy moved from less difficult to more difficult tests, up to the Hearing in Noise Test (HINT) sentences.
Figure 4 shows data for 10 sets of children who were matched for age at implant, first language, communication mode, whether they were unilateral or bilateral implant users, and their years of experience with cochlear implants. The minimum experience was 3 years and the maximum was 9 years. We also matched them on social educational status, which could include parent involvement and educational support. We felt like they were good matches.
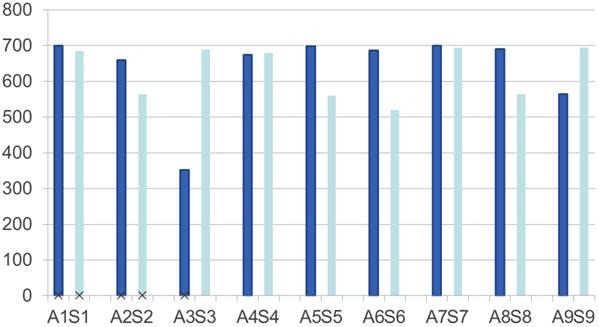
Figure 4. Data for nine matched sets of children on a battery of speech-perception tests. ANSD children= dark blue, sensorineural hearing loss match= light blue.
As you can see, the ANSD children are dark blue and the sensorineural hearing loss children are light blue. Though I do not have any statistical numbers to show you on this pilot data, we are not seeing a big difference between these children, and in some cases the ANSD children actually outperformed the children with sensorineural hearing loss. This really speaks to the question, “How do other medical conditions impact performance?” It is also supportive of the findings from a study that was recently done by Breneman and colleagues (2012) at Mayo Clinic that also matched 35 pairs of children who had sensorineural hearing loss and ANSD. They also found that these children had similar performances.
What are we Learning?
We are learning more all the time. Our latest venture has been to do more electrophysiological measures with children. I am going to explain a little more about that, and then talk about how we can best map the speech processor for a child with ANSD.
Cortical evoked potentials take a lot of forms. The one I am going to describe is the Acoustic Change Complex. There has been some published research about using cortical evoked potentials in children with ANSD (Hood, 1998; Rapin & Gravel, 2003). The setup for this test is very similar to the setup for the ABR, except you have to have a child who is awake and somewhat alert, but distracted and quiet, which is a tricky combination (Cone-Wesson & Wunderlich, 2003).
Figure 5 shows an Acoustic Change Complex for a child who has ANSD, with about a 60 dB hearing loss, and who is an excellent hearing aid user. This is a gap detection task, and this child could detect a very small gap down to about 10 ms, which is similar to what we see in children who have normal hearing. Even though they had other characteristic features of ANSD, speech recognition was good enough to use hearing aids and get very good benefit.
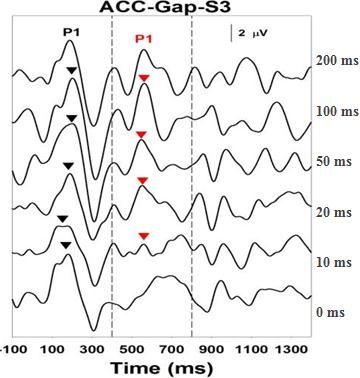
Figure 5. Acoustic Change Complex for a child with ANSD who is an excellent hearing aid user. Response can be recorded down to 10 ms.
Figure 6 is the gap detection test in another child who is a moderately successful hearing aid user. This patient has a similar level of hearing loss, and they are only able to get a gap detection response down to 50 ms.
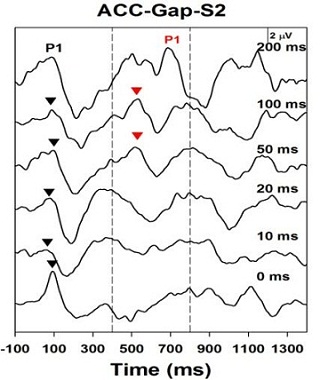
Figure 6. Acoustic Change Complex for a child with ANSD who is a moderately successful hearing aid user. Response can be recorded down to only 50 ms.
Finally, Figure 7 shows the Acoustic Change Complex results from a child with a 60 dB hearing loss, who is making no progress in language or auditory development, and who is scheduled for cochlear implant surgery. We are seeing that this patient lacks the ability to resolve gaps at all through this test. It is very consistent with the behavioral profile that we are seeing in this child.
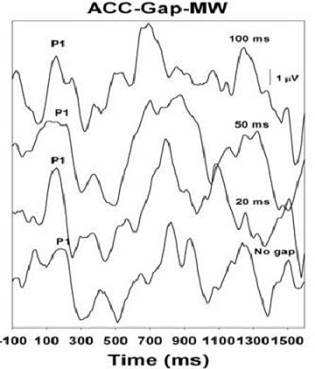
Figure 7. Gap detection results of child with 60 dB hearing loss, and with little to no language or auditory progress. No responses are recorded.
I am very privileged to work with Shuman He and John Grose, who are electrophysiologists at UNC. The following is data that Shuman presented recently at a meeting. I would like to review it with you and then refer you to her. It has not yet been published. She is preparing it for publication now. The research questions were: Do poor performers with ANSD have temporal processing deficits? Do poor performers with ANSD have spectral resolution deficits?
She included 15 children ranging from 5 years to 17 years who were all Cochlear Nucleus device users. They all had been implanted before 4.5 years. We somewhat arbitrarily made the break for determining if they were good performers or moderate performers at 70% correct using PBK words. I think that can be debated, but for the purposes of the study, that is what we used. We used a detection test format with two stimulating conditions: standard and a gap with a silent interval. The standard condition used an 800 ms biphasic pulse; the gap condition blocked the stimuli into two 400 ms bursts separated by a silent interval. The silent interval, or gap, was tested in durations of 5, 10, 20, 50 and 100 ms. We were basically looking to see if the auditory system could resolve periods of silence in between noise.
Figure 8 shows some of the results. You can see the initial onset response around 100 ms, but what we are looking for is the second P1 response that suggests that there is resolution of the gap.
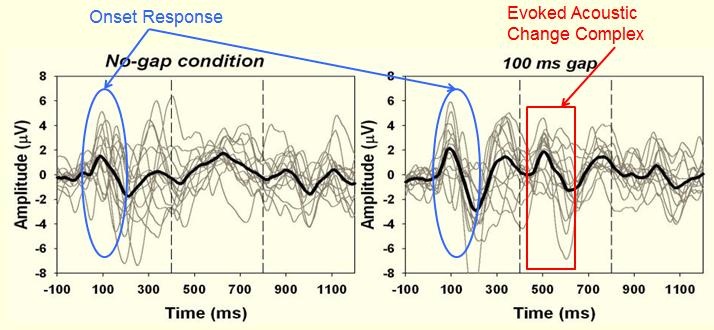
Figure 8. Standard condition with no gap (left panel) versus the gap condition showing the P1 response at approximately 500 ms.
Figure 9 shows responses from two children who achieved good speech recognition through their cochlear implants. You can see that there is gap detection resolution down to 5 ms for one child and down to 10 ms for the other child.
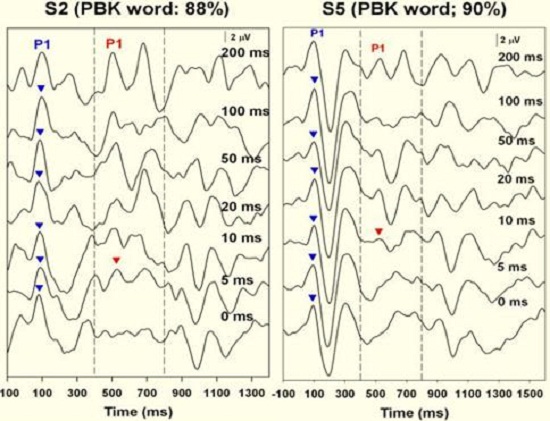
Figure 9. Results from two subjects who exhibited good word recognition after receiving their cochlear implants. The onset response is recorded (blue arrow) as well as the Acoustic Change Complex down to 5 ms in subject 2 (left panel) and 10 ms in subject 5 (right panel).
In contrast, Figure 10 shows results for children who were considered fair or poor performers with 16% and 8% word recognition scores. Their inability to resolve gaps also reflects poor resolution down to 15 ms for subject 14 and really no resolution for subject 15. When we tested a number of children, basically we saw a good correlation between the gap threshold and the PBK score. The answer to the question, “Do poor performers have temporal processing deficits?” is yes. There is electrophysiological evidence of that.
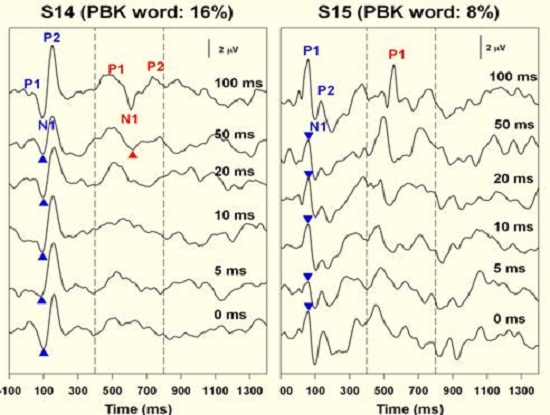
Figure 10. Results from two subjects who exhibited poor word recognition after receiving their cochlear implants. Onset responses is indicted in blue; cortical response indicated in red.
Then we asked if poor performers have spectral resolution deficits by looking at the same group of children. We presented the same standard condition of an 800 ms biphasic pulse, but this time what we called it the change condition. This condition was an 800 ms pulse train presented initially on electrode 12 and then changing to more apical electrodes, up to electrode 22, at 400 ms. We did this to see if we could see the change complex when stimulating those two electrodes.
For the good performers, we saw the Acoustic Change Complex (Figure 11) when stimulating from electrode 12 to 22, electrode 12 to 20, electrode 12 to 18, and even to the adjacent electrodes. The Acoustic Change Complex was present between electrode 12 and electrode 13 for both of these children, meaning that there was spectral resolution.
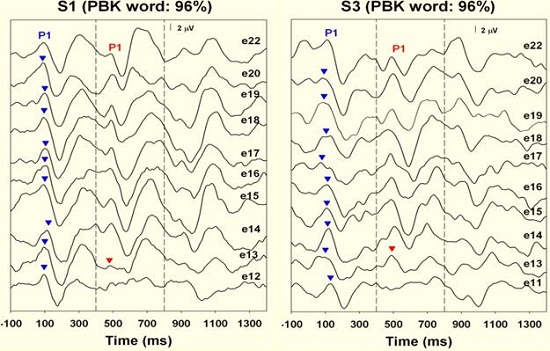
Figure 11. Spectral resolution results from good word recognition performers. Note that responses were recorded on electrode 13, adjacent to electrode 12, indicating good spectral resolution.
The fair and poor performers were not able to resolve on the channel next to electrode 12 (Figure 12). For two children, we saw a response on electrode 14, but not 13. When these results were plotted, there was a clear difference between the good and the poor performers whether or not they could resolve on the electrode adjacent to 12 or if it had to be two electrodes away. This is just pilot data, but we feel like this electrophysiologic evidence supports our hypothesis that these children do have spectral resolution deficits.
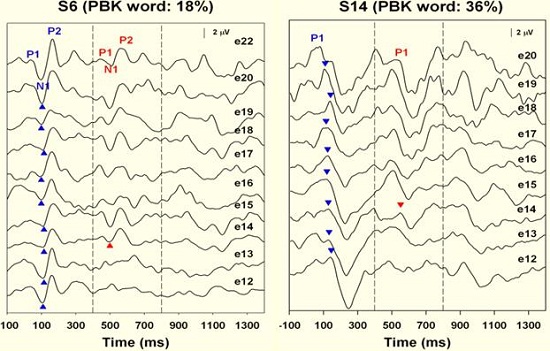
Figure 12. Spectral resolution results for fair and good performers. Responses were recorded at electrode 14, not adjacent to electrode 12, indicating less discreet spectral resolution.
We can only make these recordings after implantation, but the value may be that it can give us a prognosis for what to expect for these children, and, furthermore, if it is necessary to emphasize visual communication early on either through speech or sign support. Then we could confidently make those recommendations as part of the child’s habilitation process. On the other hand, if we see that there is good resolution, we could then be more confident about pursuing an auditory approach to communication and know that they would have the temporal and spectral ability to resolve speech and do well with that mode of input.
Optimizing Cochlear Implant Programming for Children with ANSD
What do we do with the programming for these children? Some of these data I am going to share with you were presented at the CI2011 International implant meeting in Chicago. These are practices that we have incorporated into our clinical care. Looking at each child and how much information they can give during the programming process determines how much you can implement some of these recommendations. I will review them first and then show you some of the results that we have seen that support their use.
Slow the Rate
Slowing the rate of stimulation may be one of the easiest and intuitive things that you can do for a child with ANSD to allow for that longer refractory period. There was study recently published by Pelosi and colleagues (2012) at Vanderbilt. Their hypothesis was that a slower rate of stimulation might enhance neural synchrony. They included 13 children that they studied over time, where, in fact, they did see positive results when slowing down the rate. That is something that can be easily manipulated in the software of all three manufacturers if you go to a manual mode. This is something that we often do.
Pulse Width
The second thing is to widen the pulse width. Sometimes if we have a child who has a cochlear nerve deficiency, it is not a choice; you have to widen the pulse width to get adequate stimulation. It is not unusual to have used pulse widths of 50, 75, or even 100. Of course, when we use higher pulse widths, the expectation that spectral and temporal resolution will be compromised is probably true. In these cases, these are children that were probably working very hard just to achieve sound detection because of their cochlear nerve deficiency. Even with children who do not have cochlear nerve deficiency, a wider pulse width may give them a better signal to hang on to, and we have seen some success with that.
Measure Loudness Growth
The other thing that we often do is measure loudness growth. I think this is something that we typically do with adults. We try to do this with children, but if they have no experience with previous sound or they are very young, it is a concept that is hard for them to do. When possible, being able to scale loudness and optimize the true electrical dynamic range of that patient seems to be helpful.
Pitch Rank and Eliminate Excess Channels
Pitch ranking, like measuring loudness growth, is something that we used to always do with adults, and I think we have gotten away from that in some clinics. We can try to replicate the tonotopic organization of the cochlea and take full advantage of that, so that if there are channels that are perceptually the same, we can eliminate some of those channels which will slow the rate. We can slow the rate and widen the pulse width, and it gives a little more flexibility in manipulating the parameters.
Don’t Create More Channels
Finally, we do not use strategies that create virtual channels. For Advanced Bionics patients, we would not use 120. It may be that we try this later, and I think that any of you who work with cochlear implants know that programming children is always a work in progress. As they age and are able to participate more in testing, you can get more information from them, and then at that point it may be appropriate to try virtual channels.
All of these techniques can be implemented through the programming software, but they are not the default settings. It certainly will take more time to create a program when you are manipulating those defaults.
The following are the data that we presented at the CI2011 conference (Teagle, Finley, Hatch, Park , Woodard, et al., 2011 NEED FULL REFERENCE). These were all children with the Advanced Bionics 90K device. At the time, we had 117 children using the 90K. There were 69 with whom we attempted reprogramming, and all but 3 of them preferred this alternative programming. Most of them were older children and could give us some feedback. I would like to say that there were 48 children who were using HiRes and doing just fine. These were not children who had ANSD. These were just children who were using the Advanced Bionics device.
The steps that we take when we simplify the program is to turn off Fidelity 120, use HiRes-S, pitch rank two channels at a time, if possible, turn off any of the electrodes or channels that are out of order or provide the same percept to the child, and then adjust the pulse width to about 1500 pulses per second once we know the number of electrodes. Measure T and M thresholds using tone bursts on every channel once the channels, stimulus rate and pulse width have been established. This will take quite a bit more time than your easy fit. The good news is that among all of the children with which we made these manipulations, we saw a significant improvement in the PBK scores. Again, these are not children with ANSD. They were just children who were using the 90K device who had some dramatic improvement over time in performance. Most of these children had a baseline of up to several years of cochlear implant use; the trajectory of their improvement was pretty significant once we changed to this alternative strategy.
There did happen to be some children in that group who did have ANSD. For the purpose of this presentation, I went back and pulled them out and looked at their more recent data, shown in Figure 13. You can see that not all of the children started with the modified program; some of them were on a higher trajectory at the start, so it was difficult to determine if some of the children would have ended up at a higher ending point anyway. For some of these children, after 30 months of implant use, they were still not open set-performers, and when we made the change, we saw a nice improvement over time. It is definitely a smaller set of children, but based on what we have learned from the electrophysiological testing, it made sense to make some of these changes. I think it has had an impact on their overall performance.
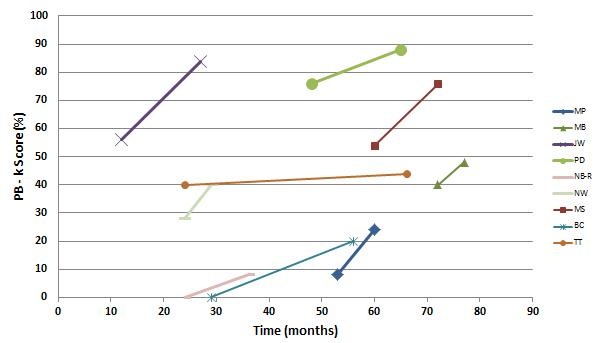
Figure 13. Subgroup of Advanced Bionics 90K users with ANSD as a function of PBK score over time of implant use in months.
Counseling
I would like to close with some general information of what we say to families when we are counseling them about ANSD. I think it is important that we demystify the diagnosis. It does not help when we are asked to tell them that this is a very strange disorder and we do not understand it. Realize that if you try to offer some replication of what their child’s hearing may sound like, you really do not know if that is a true representation, as hearing in these children varies significantly. I think we need to be very direct with parents and say that their child has an auditory disorder. We should stress that there is a range of effects that we see and that the child’s other medical conditions and the cause of the hearing loss will impact how well they do with their hearing aid or cochlear implant.
We always advocate early intervention and a team approach, and we let families know that there will be many follow-up visits involved, whether they use a cochlear implant or a hearing aid. It also may be more difficult for us to predict how well the child will do, but it is important to reevaluate progress frequently. Reevaluation will help guide decisions on communication mode. As I said, we are learning more and more about how electrophysiological measures may be predictive, but it is still very early on to use that as a counseling tool. We hope to be able to find that more helpful in the future.
We emphasize that we will only know if the implant is helpful if the child wears it and if the fit has been optimized. Optimizing the fit may take some additional appointments. The idea that the child is a work in progress should be conveyed, and also that we are going monitor, adapt and adjust continuously as the child is able to tell us more information. The parents should know that this is a team approach when you are making decisions about communication strategy.
Conclusions
ANSD is much more complicated than we originally thought. The population is very heterogeneous. It is unlikely that a single approach to management is going to meet all children’s needs. There is no evidence to support withholding hearing aid fittings from a child. We know that audibility may not ensure good speech recognition. However, unless you have audibility, you do not even have a chance at speech recognition. We really advocate that hearing aids be fit to behavioral audiograms using DSL targets. Some children will benefit from hearing aids, others will not. Regardless of the amount of residual hearing, if the child does not make sufficient progress with use of a hearing aid, then we should consider a cochlear implant. Having a complete medical work-up, including an MRI, is critical.
Once implanted, speech processor programming should be customized and individualized even at the expense of taking more time. Many children will do well with the default parameters that the software offers, but others need to be given a second look, remembering to try slowing the rate, widening the pulse width, and optimizing the tonotopic perception by eliminating channels in their map.
The use of electrophysiologic measures may be predictive, which is what our pilot data is suggesting. Finally, it is very important to use a team approach to carefully monitor a child’s progress. Visual support for communication should be discussed and considered on a case-by-case basis.
Thank you very much for the opportunity to share this information with you today. I am so pleased to see that there are so many people who are interested in this topic. I look forward to meeting and seeing you at future meetings.
Questions and Answers
Have you had any experience with AB ClearVoice and children with ANSD?
ClearVoice builds on the 120 platform. You cannot use ClearVoice unless you have a child in 120. Since we do not routinely start children who have Advanced Bionics devices with 120, we start them in HiRes-S, we have not used ClearVoice with any of the ANSD patients. We have used it with some of our other sensorineural hearing loss patients and had some good results, though.
At what point in the programming would you consider programming beyond the default parameters? Do you do that at initial stimulation?
It has been a moving target for us. I think that several years ago we started with some of the default parameters, and then we realized from the data that we could do better by changing them. We tend to start off with some of those non-defaults, such as the lower stimulus rate and wider pulse width. Sometimes the child’s anatomy will necessitate that, so we do not have a choice. We have to use a wider pulse width to get adequate stimulation. For most cases, we start with those parameters, and then if the child is not making progress, we would make some more changes. A lot of these children do not have the ability to participate in programming and getting detailed information, so we may do something like a simulated CIS map, for example, by turning off every other electrode and set at a slower rate. I cannot say that we always do the same thing. There are four audiologists in our cochlear implant program and we do consult with one another, but if we know the child has ANSD, we tend to start with those parameters. If the child is not making progress after six months, or what we would expect after even three months, then we start making changes in those parameters.
Are there any special therapy strategies or techniques that you can recommend with a child who has ANSD with a CI in one ear and a hearing aid on the other?
This is a situation that we have come across. I think there may be reason to suspect the hearing aid is not providing as good of a signal as the cochlear implant. Again, this needs to be done in diagnostic therapy, making some baseline measures, taking off the hearing aid for a period of time, and seeing if the child actually does better without the hearing aid and just the implant alone. It could be that there is some masking, some confusion, or some interaction that is not normal because the two ears are using different signals.
Do you also look at changing the default parameters for the other manufacturers’ devices?
Yes we do. With a Cochlear device, we might go to a 50 pulse width program and a 500 Hz map. On a few occasions, we have had children with ANSD that we started in the default settings and were not getting the results that we wanted, so we ended up putting them in a SPEAK map, which is a 250 Hz fixed program, and got better results. One of the things I hope that the implant manufacturers will keep in place is some flexibility in that parameter selection for clinicians, because it is really important to be able to make those changes. With the Med El system, you can also use a fixed pulse width. You set it manually. If you set it at a wider pulse width, that will slow down your rate automatically.
Have you seen children who show very little differences between unaided and aided behavioral thresholds even though speech mapping shows that the aids are set appropriately?
We have, and it perplexes me. Maybe an auditory physiologist or someone could explain it. We have seen this in a child who we have reliable hearing detection levels on. We fit the hearing aid to DSL targets, but we never get that gain that we expect. I presume that the outer hair cells are just not enough to provide the kind of gain that we would typically expect. We have seen this scenario, and I do not know what to do about it, except to say that I think it suggests that the child is a good cochlear implant candidate.
Do children with ANSD exhibit different patterns in intra and post-op neural response imaging (NRI)?
Yes. It may be related to nerve health. As part of that study that was done in 2010 (Teagle, et al., 2010), we looked at the evoked compound action potential (ECAP) response, and there was definitely a correlation between performance and presence or absence of an ECAP. But in many of those children, it could have been related to cochlear nerve deficiency. We have a number of ANSD children who have very typical looking ECAP in the absence of a cochlear malformation or cochlear nerve deficiency, and it is very similar to what we see with sensorineural hearing loss.
When would you decide that a hearing aid on one ear and a cochlear implant on another ear is not working?
I think this would have to be in diagnostic therapy by taking careful baselines and making recordings of the child, if necessary. We can obviously learn a lot about a child from their speech patterns. You can look at taking the hearing aid off, monitoring the child, and putting the hearing aid back on. It is a very individualized process. We do have a number of children who use both, but I would say that most children with ANSD that we have managed who are unilateral cochlear implant users, even if they have residual hearing on the other ear, do not prefer to use their hearing aid. It does become a decision that they or their family makes even before consulting with us. The child will reject the hearing aid after getting the cochlear implant.
References
Breneman, A. I., Gifford, R. H., & DeJong, M. D. (2012). Cochlear implantation in children with auditory neuropathy spectrum disorder: long-term outcomes. Journal of the American Academy of Audiology, 23(1), 5-17.
Buchman, C., Roush, P., Teagle, H., Brown, C., Zdanski, C., & Grose, J. (2006). Auditory neuropathy characteristics in children with cochlear nerve deficiency. Ear and Hearing, 27(4), 399-408.
Cone-Wesson, B., & Wunderlich, J. (2003). Auditory evoked potentials from the cortex: audiology applications. Current Opinion in Otolaryngology & Head and Neck Surgery, 11(5), 372-377.
Hayes, D. (2011). A brief review of aetiologies associated with auditory neuropathy. ENT & Audiology News, 19(6).
Pelosi, S., Rivas, A., Haynes, D. S., Bennett, M. L., Labadie, R. F., Hedley-Williams, A. & Wanna, G. B. (2012). Stimulation rate reduction and auditory development in poorly performing cochlear implant users with auditory neuropathy. Otology & Neurotology, 33(9), 1502-1506.
Rance, G. (2005). Auditory neuropathy/dys-synchrony and its perceptual consequences. Trends in Amplification, 9(1), 1-43.
Rance, G., & Barker, E. J. (2008). Speech perception in children with auditory neuropathy/dys-synchrony managed with either hearing aids or cochlear implants. Otology & Neurotology, 29(2), 179-182.
Rance, G., & Barker, E. J. (2009). Speech and language outcomes in children with auditory neuropathy/dys-synchrony managed with either cochlear implants or hearing aids. International Journal of Audiology, 48(6), 313-320.
Rance, G. Cone-Wesson, B., Wunderlich, J., & Dowell, R. (2002). Speech perception and cortical event related potentials in children with auditory neuropathy. Ear and Hearing, 23(3), 239-253.
Rapin, I., & Gravel, J. (2003). “Auditory neuropathy”: physiologic and pathologic evidence calls for more diagnostic specificity. International Journal of Pediatric Otorhinolaryngology, 67(7), 707-728.
Starr, A., Michalewski, H. J., Zeng, F. G., Fujikawa-Brooks, S., Linthicum, F., Kim, C. S., et al. (2003). Pathology and physiology of auditory neuropathy with a novel mutation in the MPZ gene (Tyr145->Ser). Brain, 126(Pt 7), 1604-1619.
Teagle, H. F. B., Finley, Hatch, D. R., Park, Woodard, J. S., Strader & Buchman, C. A. (2011, July). A Different Approach to Programming Advanced Bionics 90k Recipients: Return to Traditional Methods and Concepts. Paper presented at the 13th Symposium on Cochlear Implants in Children, Chicago, IL. NEED FULL REFERENCE – ALL AUTHORS?
Teagle, H. F. B., Roush, P. A., Woodard, J. S., Hatch, D. R., Buss, E., Zdanski, C. J., & Buchman, C. A. (2010). Cochlear implantation in children with auditory neuropathy spectrum disorder. Ear and Hearing, 31(3), 325-335.
Wang, N. Y., Eisenberg, L. S., Johnson, K. C., Fink, N. E., Tobey, E. A., Quittner, A. L., et al. (2008). Tracking development of speech recognition: longitudinal data from hierarchical assessments in the Childhood Development after Cochlear Implantation Study. Otology & Neurotology, 29(2), 240-245.
Cite this content as:
Teagle, Holly F. B. (2013, January). Cochlear implants for children with auditory neuropathy spectrum disorder: What are we learning? AudiologyOnline, Article #11483. Retrieved from https://www.audiologyonline.com.

