Audiology Online Contributing Editor for Adult Amplification
Director of Adult Audiology
Washington University School of Medicine
Maureen Valente, Ph.D.
Director of Audiology Studies
Washington University School of Medicine
Karen Mispagel, M.S.
Research/Clinical Audiologist
Washington University School of Medicine
Introduction
Adult patients with single sided deafness (SSD) oftentimes have difficulty a) localizing; b) understanding speech when arriving at the poorer ear; and c) understanding speech in background noise, especially when the noise arrives at the better ear. For these reasons, it is clear that patients with SSD can present a challenge to the audiologist. For adult patients with SSD, audiologists will typically provide counseling related to communication problems likely to occur and/or recommend Contralateral Routing Of the Signal (CROS) amplification to the better ear. In the past few years, however, several new fitting options have become available for patients with SSD. This paper will provide information for those audiologists exploring new fitting options for these patients.
Definition of SSD
For the purposes of this paper, SSD is defined as unaidable hearing in one ear and normal hearing levels (20 dB or better) in the opposite ear. "Unaidable" is defined as profound sensorineural hearing loss, very poor word recognition, and/or marked intolerance for amplified sounds.
Hearing Aid Fitting Process for SSD
Case history and referral
Problems associated with SSD appear to have been largely ignored by hearing health care professionals because these patients generally perform well in quiet and tend to compensate in more difficult listening situations by directing the better ear is toward the speech signal. Therefore, audiologists may not consider amplification to be required or beneficial. It is the experience of the authors that if a needs assessment were administered, oftentimes patients with SSD report significant difficulty in some listening situations.
It is important to have a clear medical and social history before considering fitting a patient with SSD with amplification. The audiologist should seek answers to some important questions prior to counseling the patient about amplification options. Information should include age, occupation, listening demands, and most importantly, motivation toward amplification. Many authors (Hodgson, 1986; Harford, 1969; Pollack, 1988; Kenworthy et al., 1990; Matkin and Thomas, 1972; Courtois et al., 1988; Harford and Dodds, 1966) have stated there is a greater likelihood of success with amplification in this group if
- a high degree of motivation is present and
- listening demands are high because of lifestyle or occupation.
If the hearing loss is sudden, waiting several weeks before pursuing amplification is suggested because approximately 65% of sudden hearing loss cases report complete or partial recovery (Rambur, 1989). Medical management of sudden SSD may include vasodilating drugs, anticoagulants, histamine, cervical ganglion blocks, steroids, inhalation therapy of 95% oxygen and 5% carbon dioxide, and calcium antagonists (Rambur, 1989). A comprehensive audiometric examination should be administered following medical management, and at the time of the hearing aid evaluation, to ensure proper candidacy.
Fitting Options
Terminology and status of hearing in the better ear
The authors have noted some confusion concerning the nomenclature for CROS fittings among audiologists. First, when the aided signal is routed to the left ear, it is important to recognize that this considered a left CROS. When the aided signal is routed to the right ear, this considered a right CROS. Second, for the purposes of this paper, a "conventional" CROS is defined as a fitting where the amplified signal is sent to the better ear via an air conducted signal (e.g., wired-analog, wired-programmable, wireless-analog or digital). Third, a "quasi-transcranial" CROS is defined as a fitting where the amplified signal is sent to the cochlea of the better ear via vibration of the skull using an air conducted signal (e.g., transcranial CROS). Finally, a "true-transcranial" CROS is defined as a fitting where the amplified signal is sent to the cochlea of the better ear via vibration of the skull using a bone conducted signal (e.g., eyeglass bone conduction, bone anchored hearing aid (Baha) or Trans Ear).
Another point must be emphasized regarding conventional CROS amplification. In the original report by Harford and Barry (1965) and subsequent reports (Harford, 1969: Harford and Dodds, 1966; Courtois et al., 1988), there is a clear recommendation that user acceptance of CROS is significantly related to the magnitude of hearing loss in the better ear. If hearing in the better ear is within normal limits, then the probability of success with conventional CROS may be limited due to patient report of adverse sound quality. If a mild hearing loss is present above 1500 Hz, then a greater probability of acceptance will be achieved. The authors' experience with CROS amplification over the past several years supports this recommendation. Very few patients with normal hearing in the better ear report that any benefit attained using CROS amplification outweighs the harshness or tinniness of the amplified sound heard in the better ear. Based on these findings, the authors have adopted a philosophy that conventional CROS amplification is not a primary choice for SSD listeners if normal hearing is present in the better ear. While the authors may recommend conventional CROS, they would not be surprised if the aid is returned for credit. On the other hand, conventional CROS becomes a primary recommendation if a slight to mild high frequency sensorineural hearing loss is present in the better ear above 1500 Hz. This fitting philosophy changes to require normal bone conduction thresholds in the better ear when considering quasi- or true-transcranial CROS options. These types of CROS fittings will be discussed later in the paper.
Conventional CROS
Wired-analog
One of the earliest attempts to improve communication for SSD patients was the CROS hearing aid (Harford and Musket, 1964; Harford and Barry, 1965; Harford and Dodds, 1966; Harford, 1969). In the earliest version, CROS amplification consisted of a microphone placed over or near the unaidable ear to pick up signals arriving at that side. The output from the microphone was wired to an amplifier, receiver, and volume control via headband. The amplified signal was delivered via tubing gently placed in the open ear canal of the better ear. Due to the presence of the open earmold in the better ear, no gain was provided below 800 Hz and only marginal gain was provided between 800 and 1500 Hz. The greatest amount of gain was provided above 1500 Hz. Therefore, patients likely to receive the greatest benefit from CROS amplification were patients with normal hearing in the better ear through 1500 Hz and a slight to mild hearing loss above 1500 Hz.
Wired-programmable with directional microphone
The wired-analog aid gave way to a wired programmable CROS with omnidirectional and directional microphone options available (Figure 1). In Figure 1, the buttons on the remote could be programmed so that one button would activate omnidirectional performance for listening in quiet, while the second button could be programmed to activate the directional microphone for better performance in noisy listening situations. A third button could activate a telecoil for improved listening over the telephone. The remaining buttons serve as a volume control as well as an on/off switch. The presence of a directional microphone provides the possibility for greater speech recognition in noise than the omnidirectional microphone available in all current wireless models (Valente et al., 1995a).
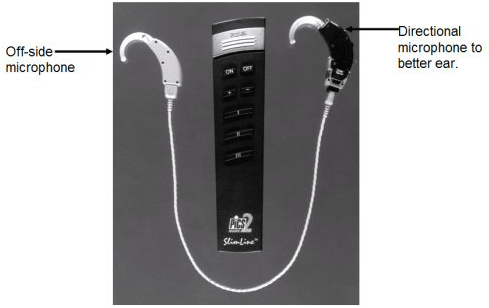
Figure 1. Example of a wired CROS hearing aid with remote control. The hearing aid on the left is an off-microphone placed over the poorer ear. The hearing aid (with dual-microphones) on the right is placed over the better ear.
While CROS amplification effectively eliminates the head shadow effect by amplifying signals from the poorer side, localization and speech intelligibility in noise still remain a problem. Some CROS users report improved localization based on differences of sound quality from the two ears. If the signal appears "natural" it may be judged to be arriving from the better side. If the sound appears "tinny" or "metallic" it may be judged to be arriving from the impaired side (Harford, 1969). Also, if the level of ambient noise is high, few users of CROS amplification report any significant benefit regardless of the side from which the signal or noise may be arising. In these environments, it may be best to counsel the patient to reduce the volume control setting or remove the hearing aids (Hodgson, 1986; Harford, 1969; Pollack, 1988).
Wireless-analog
One of the major drawbacks of the earlier CROS systems was the need for a wire connecting the output from the microphone on the impaired ear to the receiver on the better ear. To solve this problem, one manufacturer introduced a wireless BTE to ITE CROS (Figure 2). Other models included a BTE to BTE version. This wireless CROS used an amplitude-modulated carrier frequency to transmit signals from the microphone on the side of the impaired ear to the receiver placed in the better ear. Distance between the transmitter and receiver is critical (approximately 6.5 inches). For every half-inch increase in distance between the transmitter and receiver, there is a 3-4 dB decrease in gain. Several years ago the manufacturer of this wireless technology was purchased by Phonak, who decided to continue the wireless CROS line.
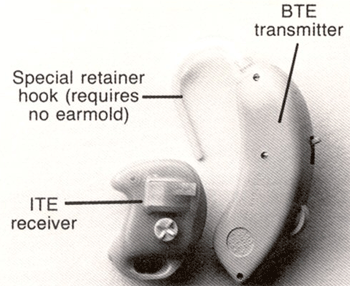
Figure 2. Example of a wireless BTE to ITE CROS hearing aid. The hearing aid on the right is the transmitter side (placed over the poorer ear) and the hearing aid on the left is the receiver side (placed in the better ear). Note. Permission to use this photo provided by Phonak, Inc.
Clinically, a major drawback of these wireless CROS systems was the limited ability to shape the frequency-gain response to provide the prescribed gain to the aided ear. Most of these models were nonprogrammable, delivered with only a low frequency tone control or low and high frequency tone controls as a means to shape the frequency response.
Wireless-digital
In the past year, several manufacturers (e.g., Interton, Phonak and Unitron) re-introduced wireless BTE to BTE (Figure 3), BTE to custom (Figure 4), and custom to custom (Figure 5) CROS aids with multichannel digital signal processing. Due to significant advances in digital signal processing (DSP) over the past decade, these models have eliminated most of the shortcomings cited above for the original wireless models.

Figure 3. Example of a wireless BTE to BTE CROS hearing aid. The image to the left is a CROSLink FM transmitter, while the image to the right is a FM receiver attached to a direct auditory input (DAI) boot that can be coupled to BTEs from over 30 different manufacturers. Note. Permission to use this photo provided by Phonak, Inc.
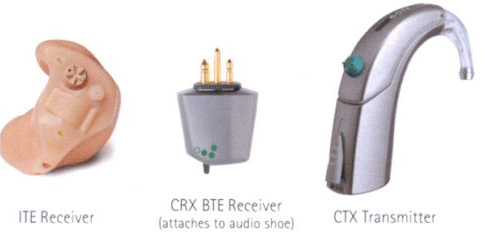
Figure 4. Example of a wireless BTE to ITE CROS hearing aid. This figure illustrates how the CROSLink BTE (right) can be ordered to communicate with a custom hearing aid with an FM receiver (left). Note. Permission to use this photo provided by Phonak, Inc.
In Figure 3, the image to the left is a CROSLink FM transmitter, while the image to the right is an FM receiver attached to a direct auditory input (DAI) boot that can be coupled to BTE's from over 30 different manufacturers. The reader can go www.phonak.com/croslink for BTE compatibility, audio shoe order numbers, CROSLink pin orientation and DPAI (designated programmable audio input) status. Figure 4 illustrates how the CROSLink BTE (right) can be ordered to communicate with a custom hearing aid with an FM receiver (left). Figure 5 is an example of an FM transmitter placed in a custom ITE aid (upper right). The transmitter sends the signal to an FM receiver (with volume control) within a custom product placed in the better ear (lower left).

Figure 5. Example of a FM transmitter placed in a custom ITE (upper right) that sends the signal to an FM receiver (with volume control) within a custom product placed in the better ear (lower left). Note. Permission to use this photo provided by Interton, Inc.
Transcranial
Quasi-transcranial
A different approach in providing amplification to SSD patients has been advocated by several authors (Sullivan, 1988; McSpaden and McSpaden, 1989; Miller, 1989; McSpaden, 1990; Chartrand, 1991). These authors suggest placing a conventional high gain/high output air conduction ITE or BTE hearing aid in the impaired ear to take advantage of the fact that the cochleas for each ear, which are contained within the temporal bones, are not acoustically isolated. That is, if an air conducted signal of high intensity is presented to the cochlea of an impaired ear, the signal will eventually be heard in the cochlea of the better ear because it will be intense enough to overcome the acoustic isolation (interaural attenuation [IA]) between the cochleas. Because the signal picked up by a microphone placed in the impaired ear is transferred to the cochlea of the better ear through the cranial structures of the temporal bone, the authors referred to this type of fitting as a transcranial CROS. As mentioned earlier, the authors of this paper prefer to refer to this type of fitting as "quasi-transcranial" because the mode of transmission to the cochlea of the better ear is via air conduction and not via the more efficient method of bone conduction that will be presented later.
The concept in developing the transcranial CROS is apparent to any audiologist who has experience in testing a patient via air conduction (earphone or insert receiver) having normal hearing in one ear and a moderate-severe to severe hearing loss in the opposite ear. The initial unmasked air conduction thresholds for the impaired ear represent the magnitude of interaural attenuation (i.e., "shadow curve"). For this patient, this represents the lowest intensity at which stimuli (non-speech or speech) will pass through the temporal bone and around the head by air conduction and be heard by the cochlea of the normal ear. In much the same way, the output (input signal plus the gain of the hearing aid) from a strong BTE or custom hearing aid placed on the impaired ear can deliver sound to the cochlea of the normal ear via bone conduction and air conduction. Signals picked up by the microphone of a hearing aid placed over or in the impaired ear can be amplified and eventually cross through the head and be heard by the cochlea of the normal ear via bone conduction (Miller, 1989).
In an effort to determine if quasi-transcranial fittings had merit, Valente et al (1995b) evaluated 12 patients with one unaidable ear in one ear and normal hearing in the opposite ear. For each patient, a strong ITE hearing aid (maximum saturation sound pressure level of 120 dB; full-on gain of 55-65 dB) with a long canal and pressure vent was fitted to the impaired ear. Four patients were experienced users of CROS amplification. Two patients had experience with an eyeglass bone conduction hearing aid placed on the mastoid process of the impaired ear. Five patients had no experience with amplification, and one patient had experience with a mild ITE hearing aid coupled to the better ear. At the end of four weeks, half of the patients felt that the ITE quasi-transcranial CROS provided significant benefit, while the other half noted little additional benefit and decided to continue to utilize their current hearing aids or not pursue amplification. For the interested reader, Valente et al (1995b) provide a method whereby real ear measures can be used to verify that the aid placed in the impaired ear provides sufficient output to exceed the interaural attenuation and be heard in the cochlea of the better ear.
It is important to remember that the acceptance rate at Washington University at that time for conventional CROS fittings for this population (normal hearing in the better ear) was approximately 10%, while the acceptance rate for the quasi-transcranial CROS is 50%. The reasons for rejection of the quasi-transcranial CROS by many of the patients were related to feedback or to a sensation of vibration generated from the hearing aid. The results of this study were encouraging. Interestingly, and of some clinical value, was the fact that the patients preferring the quasi-transcranial fit were those patients having the lowest interaural attenuation values (i.e., greater ease for the amplified signal to reach the normal ear).
True-Transcranial Bone conduction
While 30-50 dB or more of gain is necessary for the output from an air conducted quasi-transcranial CROS fitting to reach the cochlea of the better ear, minimal gain is required for a signal delivered via bone conduction to reach the cochlea of the better ear. This is because interaural attenuation for a bone conducted signal is virtually 0 dB. That is, as soon as a signal is transmitted by bone conduction to the cochlea embedded in the mastoid process of the temporal bone of one ear, it is instantaneously heard in the cochlea embedded in the temporal bone of the opposite ear. Therefore, the signal detected by the microphone placed over the anacusic ear and amplified by a bone conduction hearing aid is immediately heard in the cochlea of the better ear.
Fowler (1960) appears to be the first to advocate placement of a bone conduction hearing aid over the mastoid process of the impaired ear for patients with SSD. Usually, bone conduction aids are advocated for ears with chronic middle ear drainage or an ear canal with atresia. Since interaural attenuation for a bone-conducted signal is negligible, only one ear is required to have normal hearing via bone conduction.
Bone conduction aids are still available in eyeglass form (Figure 6). In addition, bone conduction aids are available in body type or BTE configurations, with the bone vibrator tightly held in place on the mastoid process via a headband. Just like the quasi-transcranial CROS, a bone conduction eyeglass hearing aid has a microphone, amplifier, volume control, and bone conduction receiver mounted in the eyeglass temple behind the ear with a vibrating pad projecting slightly toward the mastoid process on the side of the impaired ear. It delivers sound from that ear, routing it transcranially via bone conduction to the cochlea of the normal ear. For a patient with an unaidable ear and normal hearing in the opposite ear, the bone conduction aid can provide improved awareness and recognition of speech arriving on the side of the poor ear. However, like CROS and quasi-transcranial CROS amplification, bone conduction aids do not appear to improve localization significantly or the recognition of speech dramatically in high levels of background noise for most patients.

Figure 6. Illustration of an eyeglass bone conduction hearing aid.
In an attempt to determine the merit of fitting a bone conduction hearing aid, the first author evaluated seven patients with an unaidable ear on one side following surgical removal of an acoustic neuroma. Of the seven patients, six decided to purchase the hearing instrument (rejection rate of 14.3%) after a 30-60 day trial period. Interestingly, one patient who decided to purchase the bone conduction hearing aid had a long history of experience with CROS amplification. These seven patients reported similar improvements in communication that were described earlier for our patients using a quasi-transcranial CROS. Whereas the acceptance rate at Washington University for conventional CROS fittings for this population is 10%, the acceptance rate was 86% for an eyeglass bone conduction hearing aid.
Although this fitting was very successful with this small group of patients, a bone conduction eyeglass fitting is not without numerous obstacles. First, when the eyeglass is removed the patient will be without amplification. Second, not all unilaterally hearing impaired patients have visual impairments and therefore may not be interested in pursuing an eyeglass fitting. This problem was solved for two patients by having the optician fit ordinary glass into the eyeglass frame. Third, it is very important for the bone vibrator to fit directly on the mastoid with sufficient static pressure to deliver the amplified signal effectively to the mastoid process.
Each eyeglass bone conduction aid is available (from Starkey Laboratories) with a series of ten extension tips to place the bone vibrator directly on the mastoid. It is often necessary to make several trips to an experienced optician before the ideal placement and pressure are achieved. For some patients, the required pressure necessary for maximum benefit from this type of hearing instrument may cause irritation of the skin under the bone vibrator, discomfort, and headaches. These problems led to rejection by one of our patients, although he found the hearing aid fitting to be beneficial. In addition, audiologists tend to avoid eyeglass fittings because they assume the patient would not be interested in pursuing such a fitting.
Bone anchored hearing aid (Baha)
In 2002, the Food and Drug Administration (FDA) approved the Bone Anchored Hearing Aid (Baha) (Figures 7 and 8) by Entific Medical Systems to be fit to patients with SSD (Wazen et al, 2001; 2003; Niparko et al, 2003; Hol et al., 2004; Stenfelt, 2005). Entific was purchased by Cochlear Corporation in 2005 and the Baha device is currently available through Cochlear. Recently, the Divino digital version of the Baha was introduced (Figure 8). In the Divino, the directional microphone is encased in the unit, whereas in the previous Compact version, the directional microphone was an attached option.
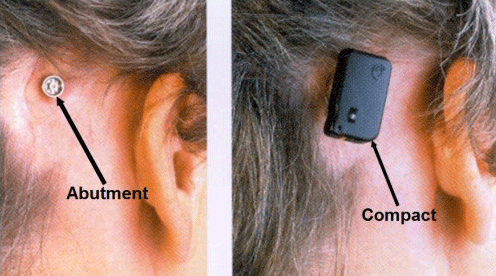
Figure 7. Left: abutment and titanium screw placed into mastoid of the right ear. Right: Compact Baha snapped onto the abutment. Note. Permission to use this photo provided by Cochlear Corporation.
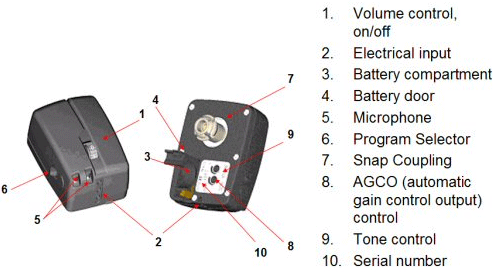
Figure 8. Illustration of the Divino Baha and the various components. Note. Permission to use this photo provided by Cochlear Corporation.
With the Baha, the patient undergoes outpatient surgery where a titanium screw and abutment are implanted into the mastoid process of the dead ear (left side of Figure 7). In the adult, it takes about three months for the implant to osseointegrate with the mastoid bone before the Baha device can be snapped into the abutment and fit (right side of Figure 7).
Currently, the Baha is available in the Divino (Figure 8) and Cordelle II. The Cordelle II provides about 13 dB greater output than the Divino and is typically utilized for those patients who report that the gain provided by the Divino is insufficient. The Cordelle has a built-in telecoil (M/T/MT switch), tone switch for low and high frequency attenuation and K-Amp signal processing where the compression kneepoint and loudness boost may be adjusted. The Divino is a two-program (one for quiet and the other for noise) device that operates on a size 13 battery and has a volume control/on/off, tone control, and output control (AGCo). The Divino is capable of connecting directly to hearing assistive technology (FM, infrared, Walkman devices, etc) via direct auditory input (DAI). Finally, a telecoil "wand" that plugs into the bottom of the Divino may be ordered as an option.
These devices transmit amplified sound directly to the skull without interference from the intermediate tissue. The Baha"¥ consists of a titanium fixture that is anchored into the skull and a percutaneous titanium abutment that is attached to the titanium fixture and penetrates the skin. Finally, a processor is connected to the protruding part of the abutment. With the Baha no tissue is present to impede the transmission of the amplified sound and the processor does not press against the skin to cause irritation.
Audiologic criteria for the Baha for SSD
Patients for whom no better alternative treatment exists may be considered candidates for the Baha if he/she:
- has better air conduction pure-tone average (ACPTA) at 500, 1000, 2000 and 3000 that is 20 dB HL or better (the closer to 0 dB HL the better).
- Is free from a generalized disease process that could result in poor wound healing.
- Is unable to use conventional air or bone conduction hearing aids.
- Is strongly motivated toward this surgical procedure.
- Is able to understand the objectives and expectations of this method of amplification.
- Is psycho-emotionally stable to maintain the hygiene of the percutaneous titanium abutment.
- Is at least 5 years old.
A fitting option that recently became available for SSD patients is the Trans Ear from United Hearing Systems (Figures 9 and 10). With this device, acoustical signals are transferred to a small bone conduction vibrator encased in an earmold placed in the ear canal of the poorer ear. Figure 9 illustrates the current full-shell version, while Figure 10 illustrates a smaller canal version. The bone conduction signal in the poor ear is then transferred to the cochlea of the better ear via bone conduction. The signal processing of this device is digital where there are low and high frequency potentiometers to shape the frequency response. This device is available with two programs with different frequency responses. The first program is for listening in quiet listening conditions, while the second program is designed for listening in noise.
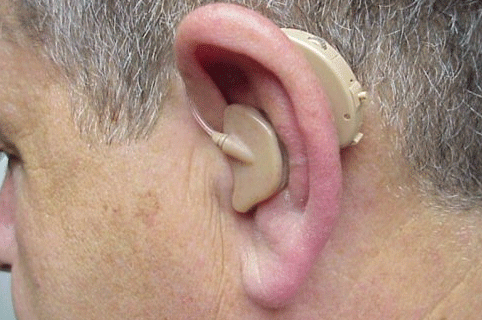
Figure 9. Illustration of the current version of the Trans Ear bone conduction BTE placed over the left ear using the full-shell design. Note. Permission to use this photo provided by Ear Technology Corporation.
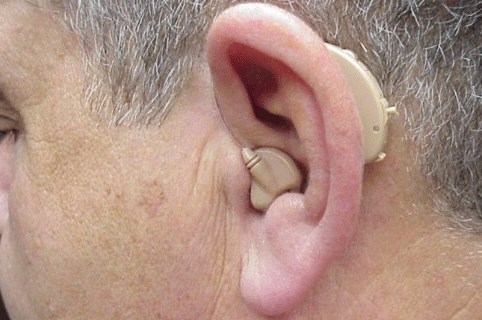
Figure 10. Illustration of the half-shell version of the Trans Ear bone conduction BTE placed over the left ear. Note. Permission to use this photo provided by Ear Technology Corporation.
The Trans Ear device was recently fit on three patients at the authors' clinic. Two were current users of the Baha, while the third had no prior experience with amplification. Unfortunately, due to problems related to feedback, the patients were not able to achieve the great amount of gain and widest possible frequency response available with this hearing instrument. According to the manufacturer, this problem is currently being addressed in an updated version that will include feedback management.
Conclusion
The primary goal of this paper was to reinforce the idea that several fitting options are available for the SSD patient. Hopefully, the fitting options reviewed within this paper will instill the concept that patients with SSD experience significant communication problems. Suggesting "directing your head to the desired signal" or recommending conventional wired CROS amplification to the better ear should not be the exclusive rehabilitative options for these patients.
The authors have gained considerable experience with the fitting options outlined in this paper and urge audiologists to consider wireless CROS, Trans Ear, Baha, quasi-transcranial CROS or bone conduction options for patients with SSD. The authors believe, and have demonstrated on numerous patients, that one or more of these options may be more beneficial in many cases than "traditional" options.
Works Cited
Chartrand, M. (1991). Transcranial or internal CROS fittings: Evaluation and validation protocol. Hearing Journal, 44, 24-28.
Courtois, J., Johansen, P., Larsen, B.V. (1998). Hearing Aid Fitting in Asymmetrical Hearing Loss. in J.H. Jensen (Ed.), Hearing Aid Fitting: Theoretical and Practical Views. 13th Danavox Symposium. (pp. 243-255) .Copenhagen: Stougaard Jenson .
Fowler, E. (1960). Bilateral hearing aids for monaural total deafness. Arch Otolaryngol, 72, 57-58.
Harford, E. (1969). Is a hearing aid ever justified in SSD? In L. Boles (Ed.), Hearing Loss-Problems in Diagnosis and Treatment. Otolaryngol Clin N Amer, 153-173.
Harford, E., Barry, J. (1965). A rehabilitative approach to the problem of unilateral hearing impairment: Contralateral routing of signals (CROS). J Speech Hear Dis, 30, 121-138.
Harford, E., Dodds, E. (1966). The clinical application of CROS. Arch Otolaryngol, 83, 73-82.
Punch, J.F. (1988). CROS revisited. ASHA, 30, 35-37.
Harford, E., Musket., C. (1964). Binaural hearing with one hearing aid. J Speech Hear Dis, 29, 133-146.
Hodgson, W.R. (1986). Special cases of hearing aid assessment, In W.R. Hodgson (Ed.), Hearing Aid Assessment and Use in Audiologic Habilitation, 3rd Edition (pp. 191-216). Baltimore: Williams and Wilkins.
Hol, M., Bosman A., Snik, A., Mylanus, E., Cremers, C. (2004). Bone-anchored hearing aid in unilateral inner ear deafness: a study of 20 patients. Audiol Neuro Otol, 9, 274-281.
Kenworthy, O., Klee, T., Tharpe, A. (1990). Speech recognition ability of children with unilateral sensorineural hearing loss as a function of amplification, speech stimuli and listening condition. Ear Hear, 11, 264-270.
Matkin, N., Thomas, J. (1972). The utilization of CROS hearing aids by children. Maico Audio Lib Ser, 10.
McSpaden, J. (1990). One approach to a unilateral "dead" ear. Audecibel, 39, 32-34.
McSpaden, J., McSpaden, C. (1989). A method for evaluating the efficacy and effectiveness of transcranial CROS fittings. Audecibel, 38, 10-14.
Miller, A. (1989). An alternative approach to CROS and Bi-CROS hearing aids: An internal CROS. Audecibel, 39, 20-21.
Niparko, J., Cox, K., Lustig, L. (2003). Comparison of the bone anchored hearing aid implantable hearing device with contralateral routing of offside signal amplification in the rehabilitaiton of unilateral deafness. Otol Neurotology, 24, 73-78.
Pollack, M. (1988). Special applications of amplification. In M.C. Pollack (Ed.), Amplification for the Hearing Impaired, 3rd Edition (pp 295-328). New York: Grune Stratton.
Rambur, B. (1989). Sudden hearing loss. Nurse Pract, 14(8), 11,14-19.
Stenfelt, S. (2005). Bilateral fitting of Bahas and Baha fitted in unilateral deaf persons: acoustical aspects. Int J Audiol, 44, 178-189.
Sullivan, R. (1988). Transcranial ITE CROS. Hear Instrum, 39, 11-12,54.
Valente, M., Fabry, D., Potts, L. (1995a). Recognition of speech in noise using a hearing aid with dual microphones. J Amer Acad Audiol, 6, 440-449.
Valente, M., Potts, L., Valente, M. (1995b). Goebel J. Wireless CROS versus transcranial CROS for SSD. Amer J Audiol, 4, 52-59.
Wazen. J., Spitzer, J., Ghossaini, S., Kacker, A., Zschommler, A. (2001). Results of the bone anchored hearing aid in unilateral hearing loss. Laryngoscope, 11(6), 955-958.
Wazen, J., Spitzer, J., Ghossaini, S., Fayad, J., Niparko, J., Cox, K., Brackmann, D., Soli, S. (2003). Transcranial contralateral cochlear stimulation in unilateral deafness. Otolaryngology-Head and Neck Surgery, 129(3), 248-254.


