Today's pediatric audiologist has a goal to identify infants with hearing loss by 3 months, and initiate intervention by 6 months (Joint Committee on Infant Hearing [JCIH], 2007). In many cases, we are successful in meeting this goal, particularly when babies are otherwise healthy and universal newborn hearing screening programs are part of a comprehensive approach to Early Hearing Detection and Intervention (EHDI). For many families, early intervention will include hearing aid fitting as part of the overall plan, yet few EHDI programs have a written, comprehensive protocol for hearing aid fitting in infancy. This means we are fitting hearing aids in early infancy more often than ever before, but also that each practitioner might use his or her own approach. This article will discuss a few ways in which this important yet challenging work is a little bit different than fitting hearing aids in young children, and a few reasons why EHDI programs might consider adopting a systematic approach.
Responsible infant hearing aid fitting needs to ensure that babies are neither over-amplified nor under-amplified, and that the hearing aids will provide comfortable yet beneficial levels of sound. What special factors might present barriers to accurate hearing aid fitting for babies in EHDI programs? In our program, we think that special attention needs to be paid to three infant-specific areas: (1) We use electrophysiology to estimate hearing rather than behavioral audiometry during the earliest parts of early intervention; (2) Babies' ear canals are small and growing, presenting ongoing challenges for fitting and re-fitting; and (3) The accuracy of infant hearing aid fitting can be enhanced by routine verification of every fitting. This article will discuss each of these areas and illustrate why they matter, using case examples.
Assessment of Infant Hearing: The great divide?
Two key developments in the hearing industry have given us the ability to successfully find babies with hearing loss and test their hearing. First, accurate, cost-effective instruments that automate evaluation of the otoacoustic emission (OAE) and/or auditory brainstem response (ABR) allow us to screen for hearing loss. Second, the use of evoked potentials for evaluating response thresholds to frequency specific stimuli let us have a substitute for a behavioral audiogram literally months sooner than would otherwise be possible. Together, these two tools let us find and test babies with enough precision that we can fit their hearing aids in the middle part of the first year of life. This is extremely exciting!
The next logical step is to move on to the hearing aid fitting. It may seem obvious how we can do this: simply enter the thresholds from the evoked potential testing into the hearing aid fitting software, right? Unfortunately, it is a little more complicated than that. You see, different procedures exist for evoked potential threshold estimation. Each procedure uses different stimuli and calibrations, which are not equivalent to the calibration used in your conventional audiometer. Audiologists who perform evoked potential assessments are quite familiar with this, but sometimes the audiologist who fits the hearing aids hasn't seen an ABR since graduate school. There have been a lot of developments in the field of evoked potentials - and this article is certainly not meant to cover them - but we would like to give audiologists who fit hearing aids a general sense of some important issues that matter for infant hearing aid fittings in this section.
The ABR has been used the longest for estimating thresholds in babies. It can be completed with broadband stimuli (clicks) or with frequency-specific stimuli (tone pips). However, case reports have shown that individual babies with unusually configured audiograms may be misdiagnosed by using click-only testing (Stapells, 2000a). To avoid this problem, it is recommended that ABR testing be done using brief tones (British Columbia Early Hearing Program [BCEHP], 2006; JCIH, 2007; Ontario Infant Hearing Program [OIHP], 2008). These tone-ABR tests give more frequency-specific results than click-ABR, avoiding most configuration problems (Stapells, 2000b). The waves they elicit, however, are less robust in appearance than a click-evoked ABR wave V, making them harder to read, particularly if the clinician has not received training and mentorship. These pros and cons may help to explain why we may still see both stimulus types being used, and why some clinics choose to use them in combination. For example, clicks are used to determine the presence or absence of auditory neuropathy spectrum disorder (ANSD) and tone pips are used for threshold estimation (BCEHP, 2006; OIHP, 2008). Figure 1 shows an example of tone-ABR threshold estimation for a baby seen in the H.A. Leeper Speech and Hearing Clinic at the University of Western Ontario. The results are shown for 2000 Hz and responses were observed at test levels of 60 dB nHL. This process gets repeated at other test frequencies (and in the other ear!) until at least one low and one high frequency threshold has been obtained and replicated.
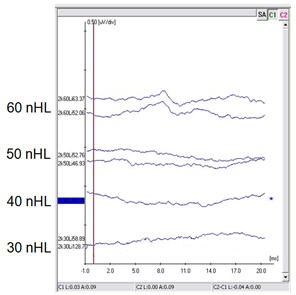
Figure 1. An example of hearing threshold estimation in an infant using ABR testing with a 2000 Hz tone pip.
Let's take this back to the hearing aid fitting for a moment. Assume that we had a complete audiogram done using the technique shown in Figure 1, including ear-specific thresholds for both air- and bone-conducted stimuli, for low (500 Hz) and high (2000 Hz) frequencies, and that the results (along with the rest of the test battery) told us that a sensorineural hearing loss had been found. However, that doesn't necessarily mean that we can type a "60 dB" threshold into hearing aid fitting software.
Why not? Well, that 60 dB threshold was measured in dB nHL, not dB HL. If that seems unimportant, consider that the differences between nHL and HL thresholds have been shown to be as high as 20 to 25 dB (Stapells, 2000b). These differences need to be subtracted from the nHL thresholds before the measurements can be used in hearing aid prescription and fitting. Otherwise, the hearing aids may be set to have too much gain, particularly in the low frequencies.
So what is the solution? There are several, with more on the horizon all the time. First, published corrections for tone-ABR measures are used to convert the thresholds from nHL to HL (Stapells, Gravel, & Martin, 1995). Or, version 5.0 of the DSL Method offers built-in or user-defined corrections from nHL to estimated HL (Bagatto et al., 2005). For either of these approaches, the correction you use should come from data that match the test procedure (stimulus type, calibration levels, etc.) that was used to test the baby. Other types of evoked potentials (some ASSR, some click-based procedures) offer built-in calibration levels, and therefore report their measurements in dB HL directly (e.g., Van Maanen & Stapells, 2009; Gorga, Kaminski, Beauchaine, & Bergman, 1993). Others have published corrections much like those for tone-ABR measures (Rance et al., 2005; Picton, Dimitrijevic, Perez-Abalo, & Van Roon, 2005). HL-based evoked potentials may be used without further correction, although note that some have been evaluated only on adults - look for infant studies to check that the procedure gives accurate threshold predictions in babies. Recently, standards for electroacoustic calibration of ABR equipment have been developed and are gaining clinical utility (ISO 389-6:2007).
To summarize, the starting point of an early hearing aid fitting for a baby is a prediction of the audiogram from an evoked potential assessment. Within the three main classes of evoked potentials (click ABR, tone ABR, ASSR), there are several subtypes, any of which may use different calibration levels. Therefore, some procedures may need some correction before they can be used to predict the audiogram in HL. A word to the wise: find out what type of procedure you (or your colleagues) are using, and whether it needs correction, before using the data to fit a baby's hearing aids. The reason for this caution is simple: missing the correction can lead to overamplification. Applying the correction is quite straightforward - Table 1 shows an example. This baby was tested with tone-ABR, calibrated according to Stapells' recommendations (see www.courses.audiospeech.ubc.ca/haplab/ThreshABR.html). Corrections are subtracted at each frequency to convert the nHL measurements to a predicted HL audiogram. This predicted audiogram was used to fit the baby's hearing aid on this ear.
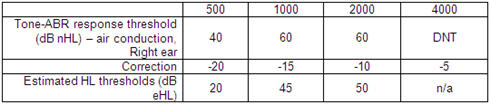
Table 1. Tone-ABR assessment results in dB nHL and their correction to estimated HL values. Correction values are those initially* used by the Ontario Infant Hearing Program, and assume ABR calibration as described in the text.
*Correction values in current use by the OIHP (not shown here) are slightly lower due to changes in equipment, calibration and experience with tone-ABR. For updated corrections, refer to Bagatto et al, 2010.
The next step: ear canal acoustics
We all know what this section is about: the real ear to coupler difference (RECD). That is true, it is, but a few new spins have come along concerning RECDs for babies: how to get them, how to predict them. We all know that the smaller ears of babies, toddlers, and children have large ear canal resonances. For hearing aid fitting, this means that a hearing aid with 57 dB of gain in a coupler will produce higher sound pressure levels in the average baby's ear than it would in the ear of an average toddler, followed by the ear of an average child, followed by the ear of an average adult. Fewer of us are aware that the same pattern exists when we assess hearing sensitivity: baby ears receive a higher level of sound at 50 dB HL than would be received in an adult's ear, particularly at the higher frequencies (Seewald & Scollie, 1999; Voss & Hermman, 2005). This means that when we do infant hearing aid fitting, the infant's ear canal plays a role both in how we define threshold and in how we define what is needed from the hearing aid. In addition to this general age-related trend, individuals of all ages vary substantially from the average, meaning that each person's ear is unique and may be poorly described unless measured directly.
The RECD can account for the effects of the infant's ear canal on the hearing aid requirements. If insert phones were used for audiometric or evoked potential testing, the RECD can also be used to account for ear canal effects during hearing assessment. For these reasons, many infant hearing aid fitting protocols mention measurement of the RECD. Typically, RECD-based corrections are automated by hearing aid fitting software - detailed information about how this is done is available elsewhere (Bagatto et al., 2005).
One of the main obstacles in clinical practice is difficulty in measuring the RECD in a particular infant. We know that RECDs vary significantly from infant to infant, child to child, or adult to adult (Bagatto, Scollie, Seewald, Moodie & Hoover, 2002; Saunders & Morgan, 2003). So, it is important to measure them whenever possible, but at the same time this can feel like a challenge - especially in babies. Clinicians may be new to RECD measurement, and/or they may be reluctant to place a probe tube deeply into the ear canal of an infant. Moving toddlers present even greater challenges. The following sections will present a review of two solutions to these problems, and assume this basic familiarity with the RECD: it consists of measuring the sound pressure level of a test signal in a 2cc coupler, followed by measurement of the same signal in the ear. The RECD is the difference between the two. For novices to this topic, detailed measurement definitions and procedures are available (e.g., Moodie, Seewald & Sinclair, 1994, user's manuals of most probe mic systems) as well as tips and tricks suitable for helping us to make this measurement with older toddlers and children (Bagatto, 2001).
A special technique for measuring the RECD in babies
There is a modified strategy for making RECD measurements in infants and toddlers. This infant-friendly approach was designed to be more feasible for use with babies, and has been shown to produce reliable and valid results (Bagatto, Seewald, Scollie & Tharpe, 2006). First, look in the baby's ear canal to determine that it is clear of debris. Second, tape the probe tube to the eartip or earmold (Figure 2) at the correct insertion depth (2 to 4 mm past the end of the eartip or mold - this took us to 11 mm from the outer edge of the eartip in four month olds). A material like sandwich wrap (i.e., Moisture Guard) was used in this example to connect the probe tube to the earpiece. Soft surgical or first aid tape can also be used and is preferred to regular tape as it will not create a sharp edge that may be uncomfortable for the infant - this can be purchased in the bandage section of most pharmacies. Third, insert the taped combination into the baby's ear canal and measure the real ear portion of the RECD, taking care not to fold or pinch the probe tube during insertion. This procedure makes it easier to measure an RECD on a baby because the taped combination of probe tube and earpiece are inserted simultaneously. This reduces the number of times that the audiologist must insert objects into the infant's ear, reducing upset to the infant and increasing the likelihood of performing the measurement while the infant is sleeping or in a quiet state. Also, the taping keeps the probe tube at a reasonable insertion depth - a feat that is hard to accomplish with confidence using the more conventional placement methods. It is unlikely, however, that this method places the probe tube at the eardrum. Despite this, it generated RECD values similar to normative data in a sample of 30 infants, indicating that it may be at least as acceptable as conventional methods for RECD measurement. An example of a measurement made with this technique is shown in Figure 3.
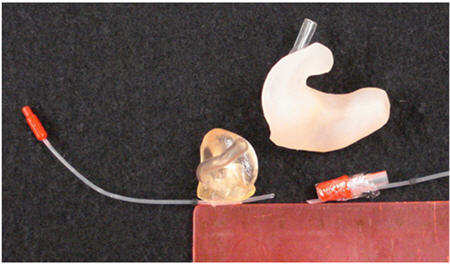
Figure 2. Probe tube microphones attached to an infant's earmold or eartip to ease the insertion of tube/eartip combination. The tubes have been attached with soft first aid tape (earmold) or Moisture Guard (eartip) so that it extends past the earmold/eartip by about 3 to 5 mm. An adult-sized earmold is shown for reference.
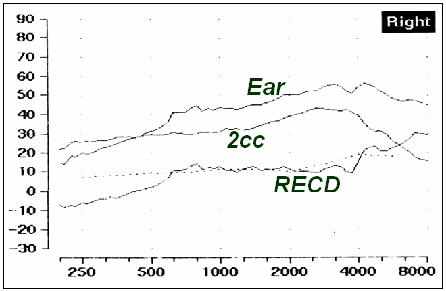
Figure 3. An example of an RECD measured on a young infant.
If you are new to RECD measures, I suggest that you learn to measure it in a few stages before attempting it with infants and toddlers. First, get some information about how to measure it, including specific instructions for your probe microphone system. Second, it helps to watch one being done by a seasoned clinician, either in person or on a video (Roush, Seewald & Gravel, 2004; Seewald, Moodie, Gravel & Buerkli-Halevy,1997). Third, get to know the procedure and your probe mic system by practicing with a coworker or cooperative adult. Once you are comfortable, begin trying it with your more cooperative patients - it is surprisingly easy to measure an RECD on most sleeping infants. Active toddlers are the biggest challenge - making good use of distractions is really helpful. It is also important to re-measure the RECD at regular intervals - it will change over time and should be incorporated into revised hearing aid fittings as the child grows. Measuring (and re-adjusting) with each new earmold is a good minimum guideline.
What if I couldn't measure the RECD this time?
Sometimes, you just can't. Sometimes you can measure it on one ear but not the other - this happens when the baby is sleeping for the first ear but rouses and is not cooperative for the second. In this case, we check our impedance results - if the middle ear is normal on both sides, it is likely OK to use the measured RECD on both sides. However, sometimes you can't measure it at all. When this happens, normative data are really very helpful. Age-appropriate predictions for the RECD have been around for years, and certainly predate EHDI programs (Feigin, Kopun, Stelmachowicz & Gorga, 1989). Newer sets of normative data are now available that tell us more about infant ear canal acoustics - for example, the biggest changes in the RECD happen in the first two years of life (Bagatto et al., 2002). These new norms have been re-analyzed and implemented into version 5.0 of DSL for use when the RECD isn't available (Bagatto et al., 2005). These new norms appear in software systems that have DSL v5.0. They are different from the older norms in a few important ways. First, they predict the RECD in age by months - so now a four month old and an eleven month old will have different predicted RECDs. Second, there are now different RECD predictions for eartips versus earmolds, and these will often be generated separately for audiometry (which often uses eartips) and hearing aid (earmolds, obviously) applications.
Moving on to the hearing aids!
Now that we know the baby's ear-specific hearing status and have some way to describe his or her ear canal acoustics, it is time to go ahead and fit the hearing aids. This assumes, of course, that the family has chosen hearing aid fitting as part of the overall intervention approach. Commonly selected hearing aids for babies will include programmability, nonlinear signal processing to allow audibility of soft sounds, and lockable features such as childproof battery doors, and lockable volume controls. Other important features are filtered pediatric-sized tone hooks to improve the physical fit to the baby's tiny pinnas, well-made soft earmolds, and a care kit that lets families check and care for the devices. Some clinics make use of FM systems in the toddler years (Moeller, Donaghy, Beauchaine, Lewis & Stelmachowicz, 1996; Gabbard, 2005), or second memories for use in noisy places like the car (rather than turning the aids off in this environment, as many parents do). Anticipating these needs in the first hearing aid selection (along with the extra 5 to 10 dB of gain that will likely be needed as ears grow) may extend the usable life of the first purchased instruments.
Once loaner or purchased hearing aids are in place, they need to be set for the individual needs of the baby. One common approach is to enter all of the baby's information into the software that programs the hearing aids, and let the automatic fitting take place. Several studies tell us that the fitting will be more accurate when the clinician routinely verifies the fitting, and makes further fine tuning to ensure that it provides the best fit to targets (Aarts & Cafee, 2005; Aazh & Moore, 2007; Hawkins & Cook, 2003; Seewald, Mills, Bagatto, Scollie & Moodie, 2008).
Let's look at a few examples of verification and fine tuning. We entered a severe audiogram for a six month old infant. For this fictional case, we assumed that the infant was tested behaviorally, and that a full audiogram was obtained. The thresholds were entered into several different hearing aid fitting software systems, and the resulting output was verified for three levels of speech and for output limiting (Bagatto et al., 2005; Scollie & Seewald, 2002). We automatically fitted each hearing aid, and verified to see if the hearing aids provided a fit to targets.
In the DSL Method, the measured hearing aid responses are compared to the baby's thresholds, targets, and predicted upper limits of comfort on one graph called an SPLogram. The hearing aids are measured on the coupler, and real ear responses are predicted based on the coupler data, the RECD, and other factors. This lets the audiologist see how the hearing aid responses relate to both audibility and comfort for the baby, acting as a surrogate for verbal feedback from the baby (Scollie & Seewald, 2002). This form of verification can be paired with validation measures that cross-check whether the expected audibility and comfort of the fitting translates into awareness of sound and development of responses to sound as would be expected. Validation measures can be questionnaires, measurement of aided thresholds, or measures of infant speech discrimination.
Figure 4 shows a comparison of the two different hearing aids fitted for this audiogram. These illustrate two of the things we sometimes see when we verify routinely. First, hearing aids vary in their response smoothness and ability to fit to targets across a broad bandwidth. In Figure 4a, the hearing aid has been fitted with too little gain/output for speech, particularly in the lowest and highest frequencies. This type of fitting would likely lead to long term problems in detecting high frequency speech sounds, perhaps leading to speech production deficits and problems in the correct use of "s"-related cues such as pluralization and possessive marking. It would be difficult for parents or teachers to attribute any of these problems to the hearing aid itself, because children with hearing loss often have difficulty detecting and using high-frequency cues in speech, even with well-fitted hearing aids (Stelmachowicz, Pittman, Hoover & Lewis, 2004). Also, the maximum power output (MPO) exceeds the infant's predicted Upper Limits of Comfort by more than 10 dB at 1000 Hz. In a real clinical situation, we would add a filter to the earhook to smooth the response (especially in the 1000 Hz region), and re-adjust the response shape for speech. Therefore, it is entirely possible that this fitting could persevere for years without adjustment, despite the fact that it is not optimized. The audiologist who routinely verifies would know, however, that the automated fitting did not set this hearing aid correctly. Simple programming readjustments to the frequency-gain response of this device would quickly resolve the problem.
In Figure 4b we see a different hearing aid, with an automatic fitting that is more successful. The MPO response is flat, smooth, and has been set to the correct level at most frequencies. The three levels of speech are quite close to target at most frequencies. It is possible that the clinician may choose to make minor adjustment to this, if the aid's flexibility permits, but generally speaking it is "in the ballpark", and provides audibility of speech peaks and loud speech to 4000 Hz, while avoiding overamplification. We are starting to see this type of successful autofit more frequently as manufacturers refine their autofit strategies to use a broadband definition of hearing aid gain and output. Not all fittings are successful though - we still routinely verify to ensure fitting consistency from child to child.
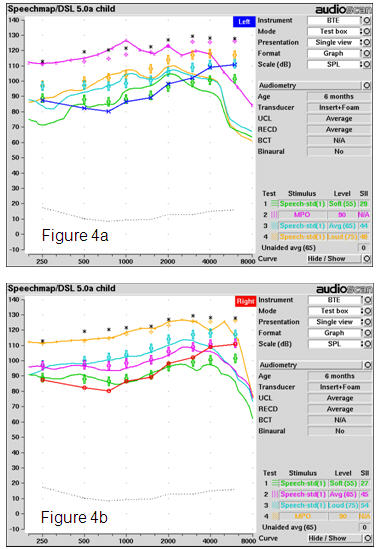
Figure 4. Verification of two different hearing aids for a 6 month old infant with a severe hearing loss: (a) Hearing aid A; (b) Hearing aid B. See text for discussion.
The conclusions? Put simply, routine hearing aid verification is still a must, and is more meaningful when the audiologist verifies the hearing aid against a validated prescription. Recently, there has been renewed interest in this type of procedure, in part because of excellent new practice guidelines (e.g., AAA, 2004; Bagatto, Scollie, Hyde & Seewald, 2010; BCEHP, 2006) and also because of speech-based verification systems that are now widely available. We hope that this trend continues - audiologists are responsible for creating infant hearing aid fittings that are beneficial and comfortable - and a high-quality electroacoustic verification can help us detect troublesome fittings before they are placed on a baby's ears.
Summary
Infant hearing aid fitting carries, in some ways, a higher level of responsibility than hearing aid fittings for older patients. The infant cannot tell us anything about the appropriateness of our fittings, and will not be able to for months or even years. We aggressively pursue early intervention for babies with permanent hearing losses, in the hopes that our services will allow them to develop better speech and language. In this article, we have tried to point out three main areas in which most EHDI programs may not have specific protocols but that likely affect the benefit that many infants will or will not receive from their hearing aids. First, the link between hearing assessment (with evoked potentials) and hearing aid fitting in infancy is not always well understood, nor is it supported by highly standardized calibration at this time. This means that audiologists who fit hearing aids to babies must ensure that corrections, if necessary, are applied to the threshold estimates that they use for early hearing aid selection and fitting. Second, the highly unique properties of most infant ear canals are likely not incorporated into most infant fittings, either because the audiologist doesn't know how to measure an RECD, or because it cannot be measured during a particular appointment. A modified, baby-friendly approach is described in this article, along with some facts about new RECD normative data. Together, we hope that these two new strategies will improve the accuracy with which we estimate infant ear canal acoustics during hearing aid fitting in the future. Third, the advent of automated hearing aid fitting seems to have persuaded many clinicians that routine electroacoustic verification is no longer necessary. We've shown two examples in which the success of automatic fitting varies considerably, and argue that routine verification is essential. Without these three protocol elements (threshold corrections, high quality RECDs, routine verification), some babies will receive appropriate hearing aid fittings while others will not. In the interest of consistency, we hope that audiologists who participate in EHDI programs will consider including these elements in their routine protocols.
References
Aarts, N.L., & Caffee, C.S. (2005). Manufacturer predicted and measured REAR values in adult hearing aid fitting: Accuracy and clinical usefulness. International Journal of Audiology, 44(5), 293-301.
Aazh, H., & Moore, B.C.J. (2007). The value of routine real ear measurement of the gain of digital hearing aids. Journal of the American Academy of Audiology,18, 653-664.
American Academy of Audiology. (2004). Pediatric Amplification Protocol. Audiology Today, 16(2), 46-53.
Bagatto, M.P. (2001). Optimizing your RECD measurements. The Hearing Journal, 54(9), 32,34-36.
Bagatto, M., Scollie, S., Hyde, M., & Seewald, R. (2010). Protocol for the provision of amplification within the Ontario Infant Hearing Program. International Journal of Audiology, 49, S70-S79.
Bagatto, M.P., Moodie, S.T., Scollie, S.D., Seewald, R.C., Moodie, K.S., Pumford, J.M., & Liu, K.P.R. (2005). Clinical protocols for hearing instrument fitting in the Desired Sensation Level Method. Trends in Amplification, 9(4), 199-226.
Bagatto, M.P., Seewald, R.C., Scollie, S.D., & Tharpe, A.M. (2006). Evaluation of a technique for measuring the real-ear-to-coupler difference (RECD) in young infants. Journal of the American Academy of Audiology, 17, 573-581.
Bagatto, M.P., Scollie, S.D., Seewald, R.C., Moodie, K.S., & Hoover, B. (2002). Real-Ear-to-Coupler Difference (RECD) Predictions as a function of age for two coupling procedures. Journal of the American Academy of Audiology, 13(8), 416-427.
Feigin, J.A., Kopun, J.G., Stelmachowicz, P.G., & Gorga, M.P. (1989). Probe-tube microphone measures of ear-canal sound pressure levels in infants and children. Ear & Hearing, 10, 254-258.
Gabbard, S. (2005). FM systems for infants and young children: A tool to optimize early listening. In R. Seewald & J. Bamford, (Eds.), A Sound Foundation Through Early Amplification: Proceedings of the Third International Conference (pp. 155-161). Stäfa Switzerland: Phonak.
Gorga, M.P., Kaminski, J.R., Beauchaine, K.L. & Bergman, B.M. (1993). A comparison of auditory brain stem response thresholds and latencies elicited by air- and bone-conducted stimuli. Ear & Hearing, 14, 85-94.
Hawkins, D., & Cook, J. (2003). Hearing aid software predictive gain values: How accurate are they? The Hearing Journal, 56(7), 26,28,32,34.
International Organization for Standardization. (2007). Acoustics - Reference zerp for the calibration of audiometric equipment - Part 6: Reference threshold of hearing for test signals of short duration.
Joint Committee on Infant Hearing. (2007). Year 2007 Position Statement: Principles and guidelines for early hearing detection and intervention programs. Pediatrics, 120, 898-921.
Moeller, M.P., Donaghy, K.F., Beauchaine, K.L., Lewis, D.E., & Stelmachowicz, P.G. (1996). Longitudinal study of FM system use in nonacademic settings: Effects on language development. Ear & Hearing, 17(1), 28-41.
Moodie, K.S., Seewald, R.C., & Sinclair, S.T. (1994). Procedure for predicting real-ear hearing aid performance in young children. American Journal of Audiology, 3, 23-31.
Ontario Infant Hearing Program. (2008, January). Audiologic assessment protocol. Ministry of Children and Youth Services, retrieved July 19, 2010 from:www.mountsinai.on.ca/care/infant-hearing-program/documents/IHPAudiologicAssessmentProtocol3.1FinalJan2008.pdf
Picton, T.W., Dimitrijevic, A., Perez-Abalo, M.C., & Van Roon, P. (2005). Estimating audiometric thresholds using auditory steady-state responses. Journal of the American Academy of Audiology, 16(3), 140-56.
Rance, G., Roper, R. Symons, L., Moody, L.J., Poulis, C., Dourlay, M., & Kelly, T. (2005). Hearing threshold estimation in infants using auditory steady-state responses. Journal of the American Academy of Audiology, 16(5), 291-300.
Roush, P.A., Seewald, R.C. & Gravel, J. (2004). Hearing care for infants: Strategies for a sound beginning. Phonak Video Focus 4. Stafa, Switzerland: Phonak.
Saunders, G.H. & Morgan, D.E. (2003). Impact of measuring threshold in dB HL versus dB SPL on hearing aid targets. International Journal of Audiology, 6, 319-326.
Scollie, S.D., & Seewald, R.C. (2002). Electroacoustic verification measures with modern hearing instrument technology. In R.C. Seewald & J.S. Gravel, (Eds.), A Sound Foundation Through Early Amplification: Proceedings of the Second International Conference (pp.121-137). Stäfa Switzerland: Phonak.
Seewald, R., Mills, J., Bagatto, M., Scollie, S., & Moodie, S. (2008). A comparison of manufacturer-specific prescriptive procedures for infants. The Hearing Journal, 61(11), 26, 28, 30, 32, 34.
Seewald, R.C., Moodie, K.S., Gravel, J.S., & Buerkli-Halevy, O. (1997). Pediatric hearing instrument fitting. Phonak Video Focus 2. Stäfa, Switzerland: Phonak.
Seewald, R.C., & Scollie, S.D. (1999). Infants are not average adults: Implications for audiometric testing. The Hearing Journal, 52(10), 64-72.
Stapells, D.R. (2000a). Frequency-specific evoked potential audiometry in infants. In: R.C. Seewald (Ed.), A Sound Foundation through Early Amplification: Proceedings of an international conference (pp. 13-32). Stäfa, Switzerland: Phonak.
Stapells, D.R. (2000b). Threshold estimation by the tone-evoked auditory brainstem response: A literature meta-analysis. Journal of Speech-Language Pathology & Audiology, 24, 74-83.
Stapells, D.R., Gravel, J.S. & Martin, B.E. (1995). Thresholds for auditory brain stem responses to tones in notched noise from infants and young children with normal hearing or sensorineural hearing loss. Ear & Hearing, 16, 361-371.
Stelmachowicz, P.G., Pittman, A.L., Hoover, B.M., & Lewis, D.E. (2004). The importance of high-frequency audibility in the speech and language development of children with hearing loss. Archives of Otolaryngology Head Neck Surgery, 130, 556-562.
Van Maanen, A., & Stapells, D. (2009). Normal multiple ASSR thresholds to air-conducted stimuli in infants. Journal of the American Academy of Audiology, 20, 196-207.
Voss, S.E., & Hermman, B.S. (2005). How does the sound pressure generated by circumaural, supra-aural, and insert earphones differe for adult and infant ears? Ear & Hearing, 26, 636-650.



