Editor's note: This text-based course is a transcript of the webinar, Intra- and Post-Operative Electrocochleography in Cochlear Implants.
Learning Outcomes
After this course learners will be able to:
- Describe electrical potentials measured using electrocochleography.
- Explain the intra-operative application of electrocochleography during cochlear implant surgery.
- Describe the post-operative application of electrocochleography in cochlear implant patients with residual hearing.
Introduction
Cochlear implants are considered a class three medical device that is used to restore hearing sensation and speech perception in patients who have varying degrees of hearing impairment. There are three approved cochlear implant manufacturers approved by the United States FDA whose devices are going to be implanted in patients, Advanced Bionics, Cochlear, and Med-EL. Each implant manufacturer has some type of a processor that goes behind the ear, or maybe all-in-one piece processor which includes a microphone. The processor uses a microphone to capture the sound from the listening environment. Then that sound is dubbed, goes through some signal processing chain, and the cable and coil, or the headpiece is communicated to the implant that is placed under the skin. The implant electronics then takes that signal and sends it to the auditory nerve using the electrode array that is placed inside the cochlea.
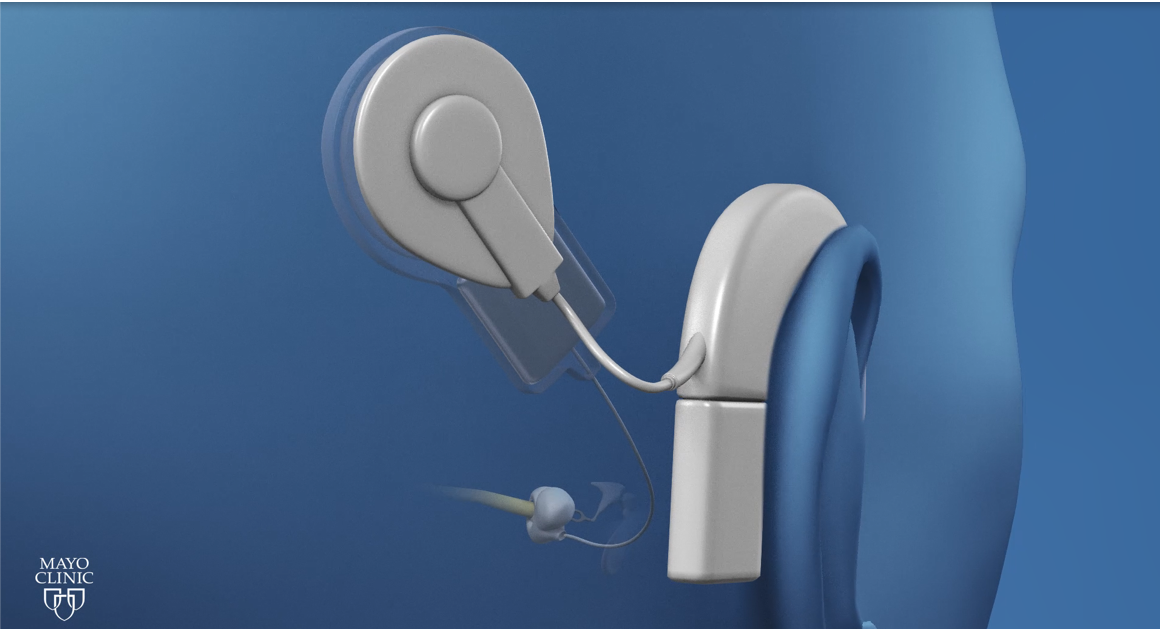
Figure 1. Cochlear implant.
Cochlear Implant Candidacy
Over the years since this technology has become available, the candidacy criteria have changed. About 10-15 years ago, it was easy to ask a question of who qualifies for a cochlear implant. The answer would be simple; patients with severe to profound hearing loss get a cochlear implant. In the last few years, the criteria have significantly changed, and more patients with significant residual hearing, not only in the low frequencies but also in the high frequencies, have started receiving cochlear implants. From our experience over the past decade, we have learned that these patients who have partial hearing loss and receive a cochlear implant receive significant benefits in terms of speech understanding and music perception, using their cochlear implantation. If we can preserve the residual hearing in these patients and amplify that ear along with the cochlear implant, then the combined acoustic and electric hearing provide the best cochlear implant outcome in terms of sound localization, speech perception, and music appreciation.
Loss of Residual Hearing During Implantation
Some patients in the expanded candidacy criteria who receive a cochlear implant show a decrease or complete loss of residual hearing after surgery. In other words, these patients have some amount of residual hearing before the surgery. After implantation, whether it is immediately after the surgery or later on, they end up losing their residual hearing. From our experience in the last 10-15 years, we have learned that several intra-operative factors could cause cochlear trauma while placing the electrode. That is, when the electrode is being placed in the cochlea, it may lead to some sort of a cochlear trauma and which can lead to loss of residual hearing. Or there might be other post-surgical factors where recovery from the surgery or the inflammation after the surgery can lead to some loss of residual hearing. So there are many intra-operative, as well as post-surgical factors that contribute to the loss of residual hearing after cochlear implantation.
Electrode Insertion
In regards to the placement of the electrode, the cochlea has three compartments shown in Figure 2. The bottom compartment is called the scala tympani. The top compartment is called the scala vestibuli. The compartment in the middle, which houses the organ of Corti, which we can see the hair cells, tectorial membrane, and basilar membrane, is known as the scala media. Ideally, the entire electrode array should be placed into the scala tympani, that is in this bottom compartment. If electrode insertion is very close to the basilar membrane, this can lead to loss of residual hearing (Roland et al., 2005). Now, imagine trying to place a tin wire in a snail-like structure, we have no idea of where the electrode is going. We have very little control over how the electrode is being placed into the cochlea. So during the placement of the electrode, if there is any kind of an insertion trauma, to the basilar membrane, whether the electrode forks into the basilar membrane or the electrode pierces through the basilar membrane and translocates from the scala tympani into the scala of vestibuli, both these types of trauma can lead to loss of residual hearing.
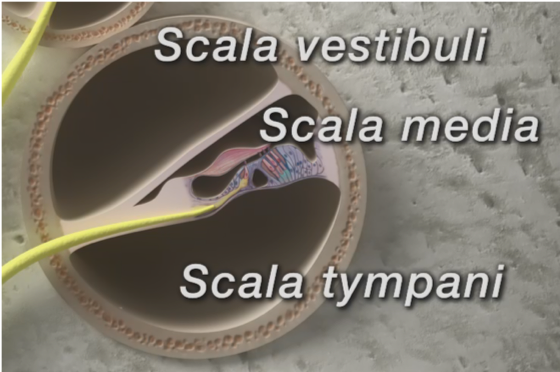
Figure 2. Cochlea.
Holden and colleagues found there are several patients who can have electrodes that translocate from scala tympani into the scala vestibuli (Holden et al., 2013). These patients perform significantly poorer in terms of their speech understanding as compared to patients who have their entire array placed into the scala tympani. I think there is very compelling evidence to say that ideally, the whole electrode array should be placed into the scala tympani and without any cochlear trauma. Now, with the current technology, we are unable to visualize the electrode location during the placement of the array.
Electrocochleography (ECOG)
This is where the role of an audiologist becomes vital. Electrocochleography (ECOG) is a technique used to measure electrical potentials from the cochlea and auditory nerve. We can utilize this technique to possibly monitor cochlear trauma and guide electrode placement during cochlear implant surgery. During this course, we will go into depth about what is electrocochleography, what are the potentials we can measure using electrocochleography? How are these potentials measured during surgery? And how are they used to monitor implant electrode placement? Post-operatively we will also look into how these potentials can be used to rehabilitate patients with cochlear implants.
Evoked Potentials Measured with ECOG
Stimulus. When we say we are going to use electrocochleography to measure electrical potentials, we first need to generate and record the potential. The electrical potential from the cochlear or the auditory nerve can be generated using a brief acoustic stimulus such as tone burst or clicks or an electrical stimulus like we routinely use during NRT, NRI, or ART measurement in cochlear implants. These are measurements we perform immediately after the electrode placement and also post-operatively to make sure that the electrical signal is reaching the auditory nerve.
Recording electrodes. Now, once we generate these potentials, whether we generate it acoustically or electrically, we need to record these potentials. These potentials can be recorded using electrodes that are placed on the surface of the scalp or using tip-trodes. The other type of electrodes that can be used to perform these measurements are transtympanic or sometimes referred to as extracochlear electrodes. Sometimes we place an electrode on the promontory to pick up these signals as well. With the advancement in the cochlear implant technology, we have been able to measure these potentials using the cochlear implant electrodes. That is, we do not need any scalp electrodes, or any extracochlear electrodes to perform these measurements.
When we present a stimulus during ECOG, depending on the type of stimulus and filtering mechanism employed, we can measure the following four evoked potentials:
- Cochlear Microphonics (CM) – Outer hair cells (ongoing response)
- Auditory Nerve Neurophonics (ANN) – Auditory nerve (ongoing response)
- Summating Potentials (SP) – Hair cells (ongoing response)
- Compound Action Potential (CAP) – Auditory nerve (onset – offset response)
SP and CAP are generally used to measure patients with Meniere's. Cochlear microphonics are assumed to be generated from the outer hair cells or mostly the hair cells and the supporting cells. Auditory nerve neurophonics, as the name indicates, comes from the auditory nerve. The summating potentials come from the hair cells, and the compound action potential is from the auditory nerve. One thing to remember is that the cochlear microphonic, ANN, and SP are the ongoing responses for the application in a cochlear implant. In other words, the stimulus and potential are presented and recorded at the same time.
Now let's discuss what we do during ECOG measurement as it relates to cochlear implants. During the ECOG measurement, we use an acoustic tone burst. Even though we are dealing with cochlear implants, we use an acoustic tone burst, and this is primarily done in patients who have some level of residential hearing in the ear that is going to be implanted.
Cochlear Microphonic
Figure 3 shows a waveform representation of a 500 Hz stimulation with condensation polarity. This is a cochlear microphonic signal. We are presenting this sinusoidal stimulus, and at the same time, we are recording an evoked potential from the cochlea. In this case, the cochlear microphonic looks the same as that of the stimulus and is shown in green.
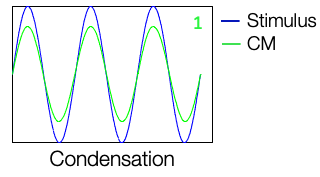
Figure 3. Condensation polarity of CM.
In Figure 4, instead of the condensation polarity, we use the rarefaction polarity. That is opposite in polarity as compared to the condensation phase and the stimulus, and the cochlear microphonic recorded from, during this presentation also looks just like the stimulus.
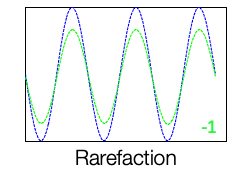
Figure 4. Rarefaction polarity of CM.
We use an alternating polarity. That is, we go back and forth between condensation and rarefaction polarity, and we keep doing this while acquiring the stimulus. If we decide to add the evoked potential measured during the condensation phase and the rarefaction phase, it will cancel out. They cancel, and we get a flat line, that is, we do not get any response shown in Figure 5.
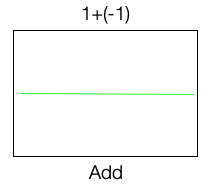
Figure 5. Alternating polarity.
However, if you take the two and now subtract one from the other, we get a nice cochlear microphonic signal. Thus instead of adding, we are going to subtract it. A cochlear microphonic is derived by subtracting the response for one phase from the other. If we add the response of one phase to the other, we end up losing the cochlear microphonic signal shown in Figure 5.
Auditory Nerve Neurophonic (ANN)
The auditory nerve neurophonic is also a phase-locked response. Remember, there is phase locking in the auditory nerve. The nerve responds in a phase-locked manner to one particular phase. When we present the rarefaction polarity, we get a similar response that is locked to another phase. When we add these two, we get what is called an auditory nerve neurophonic (ANN). Now, if we subtract the two responses, we end up getting a flat line.
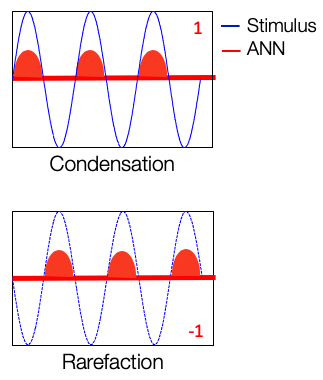
Figure 6. Auditory Nerve Neurophonic (ANN).
We are presenting a condensation phase, and to that phase, we are going to acquire the responses for cochlear microphonic (CM) and auditory nerve neurophonic (ANN). Even though they are separated in Figure 7, they are not going to be separated in the acquisition stage. We are going to do the same thing for the rarefaction phase and will acquire the responses to these two. If we subtract the responses of the one phase from the other, we get the cochlear microphonic. If we add these two responses, we get the auditory nerve neurophonic. So by definition, cochlear microphonic and auditory nerve neurophonic is nothing but the way we add and subtract the responses acquired using this electrocochleography technique. This helps us differentiate within the cochlear microphonic or the outer hair cells and the auditory nerve neurophonic of the auditory nerve.
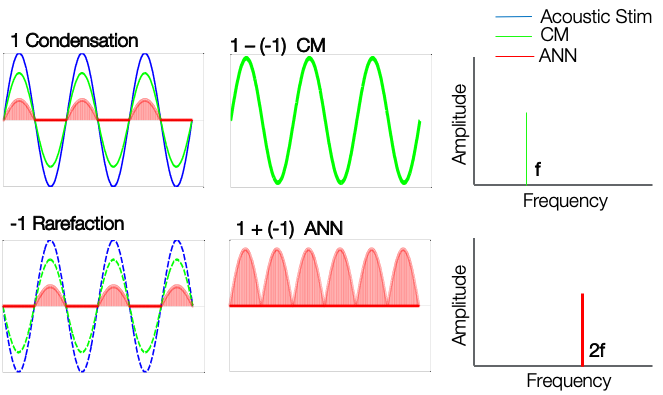
Figure 7. CM and ANN.
One important difference between these two responses is that the cochlear microphonic frequency is the same as the stimulus frequency. Whereas the auditory nerve neurophonic is two times the frequency of the stimulus. For example, for a 500 Hz stimulus, the cochlear microphonic will also have a frequency of 500 Hz. Whereas auditory nerve neurophonics is going to have a frequency of 1000 Hz.
ECOG During Cochlear Implant Surgery
In a patient who has significant residual, low-frequency hearing, for example, we can present a 500 Hz stimulus at a somewhat loud level. That stimulus is going to stimulate the apical region of 500 Hz inside the cochlea. That stimulus is going to generate cochlear microphonics. Cochlear microphonics is generated from the outer hair cells, so we measure the signal using the cochlear implant electrodes. How do we do that? The array of electrodes at the most apical region will be the recording electrode, and the case is used as the ground electrode. We record the responses between the active electrode and the ground electrode. Similarly, we record the responses within the active electrode and the case electrode. In cochlear implants, we do not use surface electrodes to perform electrocochleography because we want to do this during surgery. So we use the implant electronics as the recording electrodes and the other electrode as the ground electrode.
Remember, we want the electrode array to be placed entirely into the scala tympani. We don't want the electrode to go through the basilar membrane and cause any trauma to the surviving hair cells in the cochlea. During the surgery, we present a 500 Hz stimulus that generates cochlear microphonics. The 500 Hz stimulus is going to stimulate the hair cells in the 500 Hz region of the cochlea and generate a cochlear microphonic. When the electrode is introduced from the round window, we use this most apical electrode as the recording electrode, and we measure cochlear microphonic. As this electrode starts approaching the 500 Hz location in the cochlea, we should start seeing a gradual increase in the cochlear microphonic amplitude. The idea is that if this electrode pierces the basilar membrane and causes destruction of the hair cells, then we are going to see an immediate drop in this cochlear microphonic amplitude. This amplitude is going to be used as a guide during the placement of a cochlear implant array.
Intra-operative ECOG Set-Up
What does this all look like in the operating room? Without going too much into detail, I thought I'll give you a flowchart of how it's done shown in Figure 8. First, we have a tone generator. If you want to present a 500 Hz stimulus, we need a tone generator that is going to generate an acoustic tone burst, such as 500 Hz. That is going to be presented through an insert earphone tube into the ear canal. This tone generator is synchronized with software that is going to measure ECOG and do all the addition, subtraction, and display the cochlear microphonic amplitude. This electrocochleography software is connected to the POD or the CPI-3 processor and the cable, headpiece, and coil. This talks to the implant and captures the responses from the electrode array.
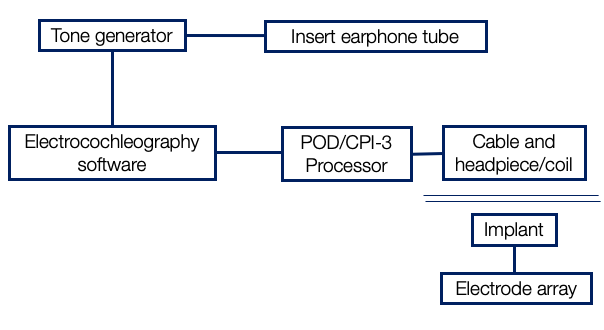
Figure 8. Inter-operative set up.
Insert earphone set-up. Figure 9 shows a typical insert earphone. The black stem of the insert earphone can be kinked, and that will prevent the 500 Hz stimulus from being delivered in the ear canal. It is essential to make sure that there is no kinking in the stem of this foam plug. Once we take this yellow foam plug and put it in the patient's ear canal, we cover the ear canal concha with something known as bone wax. We take this bone wax, and we cover it because, at some point, they are going to wash this whole region with betadine, and we don't want any liquid or betadine entering the ear canal. If there is fluid in the ear canal, our stimulus of 500 Hz will simply not make it to the tympanic membrane, and then we may not get any responses.
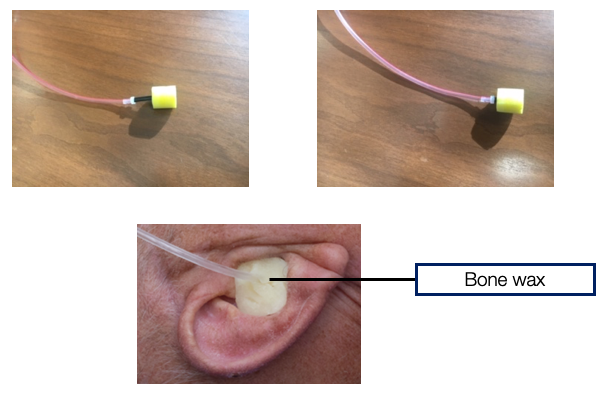
Figure 9. Insert earphone set up.
OR set-up. The surgeon is going to take the pinna and fold it over to place the electrode and measure cochlear microphonic suiting surgery shown in Figure 10. You can see the insert earphone tube, but you don't see the foam plug because it's in the ear canal and covered. The surgeon has done the facial recess, made a pocket, and placed the implant inside the pocket. The electrode array is about to be placed in the cochlea. Just before that happens, the coil and headpiece are put into a sterilized ultrasound sleeve that is shown in Figure 10. Then this coil is placed over the magnet. Our software has started talking with the implant so that when the most apically electrode is being placed, we can begin measuring cochlear microphonic.
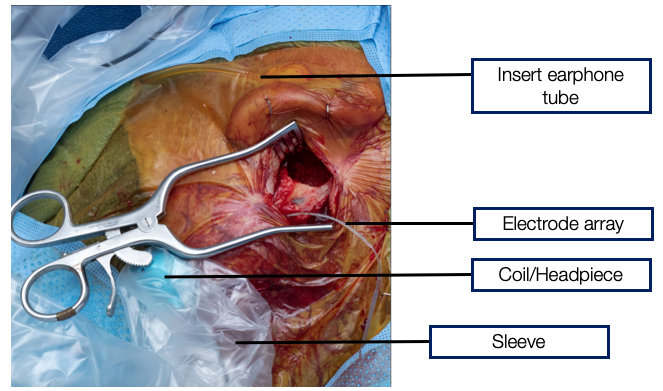
Figure 10. OR Set up.
Intra-operative ECOG Monitoring
I would like to take you into the OR where we measure the cochlear microphonic while the electrode is being placed in the cochlea by the surgeon. Here, you can see the surgical screen or this monitor that we see that is synchronized in time with cochlear microphonic measurements. Here is the amplitude of the cochlear microphonic that is measured.
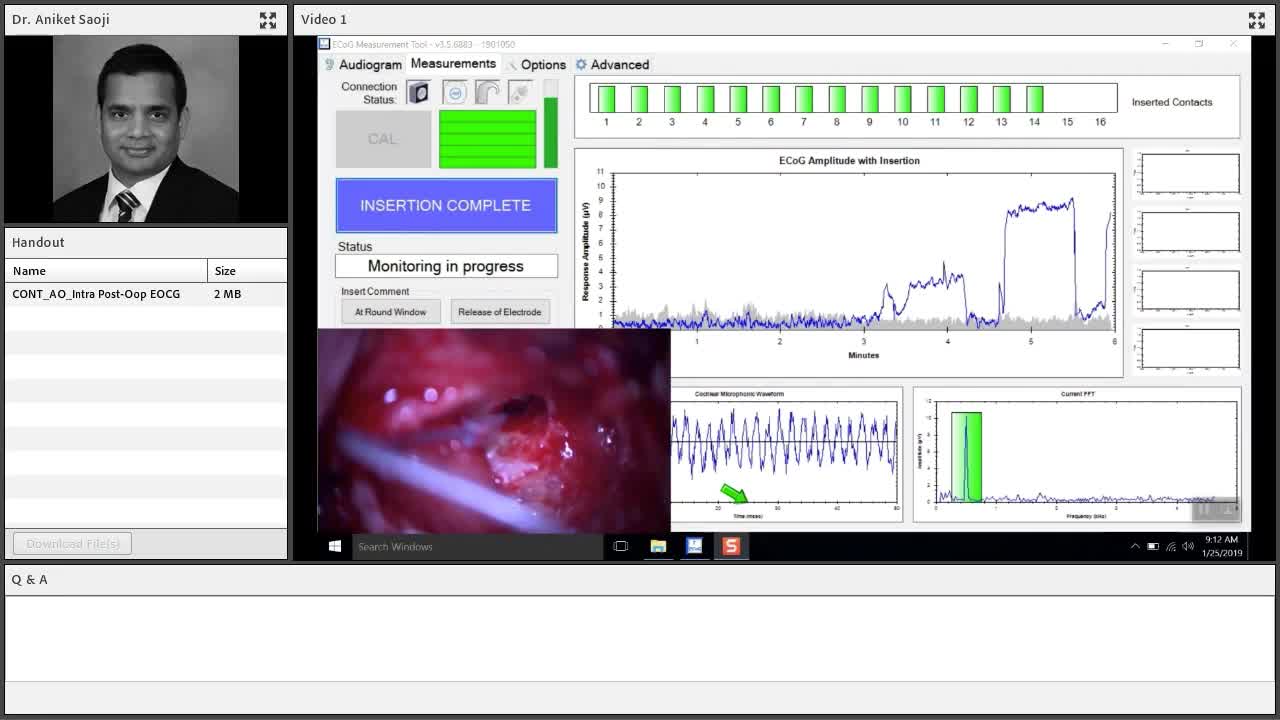
There are different ways of describing these patterns, and cochlear microphonic amplitude increases and decreases. What is significant? What is not significant? I don't think I'll be able to cover all of those things in today's presentation, but I thought I could point you to a couple of articles. These articles discuss how electrocochleography during placement of the electrode is predictive of the final scalar location. We want it to be in scala tympani and not the scala vestibuli. Also covered are the different patterns and what do those patterns of amplitude tracings mean when we are monitoring cochlear microphonic during cochlear implantation. Both of them are very nice articles and give us an in-depth understanding of how this can be used during surgery.
- Intra-Cochlear Electrocochleography During Cochlear Implant Electrode Insertion Is Predictive of Final Scalar Location. Koka K, Riggs WJ, Dwyer R, Holder JT, Noble JH, Dawant BM, OrtmannA, Valenzuela CV, Mattingly JK, Harris MM, O'Connell BP, Litvak LM, Adunka OF, Buchman CA, Labadie RF. Otol Neurotol. 2018 Sep; 39 (8):e654-e659. PMID: 30113557.
- Patterns Seen During Electrode Insertion Using Intracochlear Electrocochleography Obtained Directly Through a Cochlear Implant. Harris MS, Riggs WJ, Giardina CK, O'Connell BP, Holder JT, Dwyer RT, Koka K, Labadie RF, Fitzpatrick DC, Adunka OF. Otol Neurotol. 2017 Dec; 38 (10):1415-1420. PMID: 28953607.
Post-op ECOG Monitoring
During post-operative monitoring, we can measure something called an ECOG audiogram. I think the real word is a cochlear microphonic audiogram. That is, you systematically decrease the 500 Hz stimulus presentation level and find the lowest level at which we can measure a response and call it a cochlear microphonic audiogram. In Figure 11, we have 20 patients from one of our papers with my colleagues (Koka, Saoji, and Litvak, 2017). The black symbol shows the audiogram at different frequencies. The frequency on the X-axis, the amplitude, and dB HL on the Y-axis. The response in red is the cochlear microphonic audiogram measured. In green is the auditory nerve neurophonic audiogram, which we can ignore for this presentation. The cochlear microphonic audiogram very nicely follows the pure tone thresholds. These are pure tone thresholds measured in the implanted ear. That is the ear that had significant residual hearing before surgery. That ear was implanted, and after implantation, we were able to preserve residual hearing.
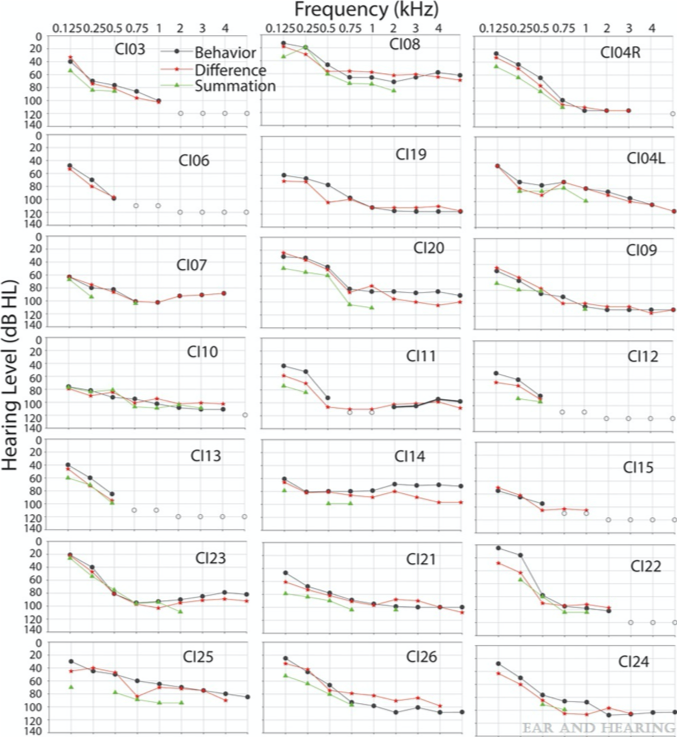
Figure 11. Koka, Saoji, and Litvak (2017).
Remember, the difference between the two stimuli and responses for the two phases is the cochlear microphonic. There is a very high correlation between these measurements. I just wanted to show you how right after the electrode placement, we can measure an audiogram and how quickly and fast we can do that. We know that we can quickly measure an audiogram after surgery. Once a patient has cochlear implant surgery, they have lots of fluid in the middle ear anywhere between two weeks to six or eight weeks sometimes. If they have fluid, we cannot measure a good audiogram. Then we sometimes rely on bone conduction thresholds. There are some vibrotactile responses to consider in that case. Testing two or three months after surgery to get a good post-operative audiogram would be the best time. Certainly, we were able to preserve these thresholds during surgery, which possibly suggests that we did not cause much cochlear trauma. Although it's all an indirect measure, there is no way to visualize and confirm that. Because post-operatively, during the recovery phase, something goes wrong occasionally. Maybe inflammation, apoptosis, or some kind of a process that is going on that adversely affects hearing preservation.
Summary
Post-operative ECOG measurements can be used as an objective tool to:
- Predict pure tone thresholds in cochlear implant patients with post-operative residual hearing (Koka et al., 2017).
- Reliably measure air-bone gap in cochlear implants patients (Koka et al., 2017).
- Measure electric and acoustic interaction in cochlear implants (Koka and Litvak, 2017).
- Determine the location of the cochlear implant electrode along the cochlear space.
I want to discuss other emerging applications of these post-operative ECOG measurements. We covered how pure tone thresholds can be predicted using the cochlear microphonic threshold measurement. Sometimes we see these patients who have developed the new air-bone gap or conductive hearing loss after surgery. These patients did not have conductive hearing loss before surgery. When they develop conductive hearing loss after surgery, a lot of times, we question whether the bone conduction responses are true or whether they are vibrotactile. Post-operatively, we can measure cochlear microphonic or ECOG responses for air-conduction stimuli as well as bone conduction stimuli. Although sometimes we do get a big artifact. In 2017, Koka and colleagues measured air-bone gap in patients reliably using cochlear microphonic measurements. Because when we measure cochlear microphonic, the only way we can get a response is if the stimulus that we are presenting reaches the basilar membrane and stimulates the hair cell. If there is a vibrotactile response, then we will not be able to measure any cochlear microphonic thresholds.
We are now stimulating electrically as well as acoustical in the same ear. It is quite possible that we are going to have some interaction between the electric and acoustic stimulus that is presented simultaneously in that ear. Which is undesired, leads to distortion, and could potentially harm the ear. So if we can measure cochlear microphonic using acoustic stimulus and then measure the same cochlear microphonic again while we are presenting the electric stimulus, we can figure out electric and acoustic interaction. Again, this is a newly emerging tool, a new application of inner electrocochleography in cochlear implants. I think there are now a couple of publications that have tried to measure electric and acoustic interaction in the implanted ear. I have listed these in the references for you to find out more information.
One thing that we have just started to begin exploring is to determine the location of the cochlear implant electrode along the cochlear space. What do I mean by that? We place so many electrodes in the cochlea, not just one. Electrode 1 might be closer to the 500 Hz, Electrode 5 may be closer to the 1000 Hz, Electrode 10 may be closer to the 2000 Hz, etc. There is no way for us to find out which electrode is located at which frequency region inside the cochlea. So if we can measure cochlear microphonic for different frequencies 250 Hz, 500 Hz, 1000 Hz, and 2000 Hz pure tone stimulus and so on, and measure and change the recording electrode and vary the recording electrode inside the cochlea. We should be able to predict which electrode is located at which frequency region inside the cochlea. That should help us determine the frequency allocation tabled in cochlear implants.
References
Citation
Saoji, A. (2020). Intra- and post-operative electrocochleography in cochlear implants. AudiologyOnline, Article 26961. Retrieved from https://www.audiologyonline.com


