Sumitrajit Dhar, Ph.D., The Roxelyn and Richard Pepper Department of Communication Sciences and Disorders, Northwestern University, Evanston, Illinois;The Hugh Knowles Center for Hearing Research, Northwestern University, Evanston, Illinois
Introduction
Although the fact that healthy ears make sounds had been recognized as early as the mid eighteenth century, it was not until David Kemp recorded otoacoustic emissions (OAEs) from his own ears (Kemp, 1978) that the scientific world took notice of the phenomenon. It would be another decade before clinically useful instrumentation would become available, and audiologists would begin using OAEs as a diagnostic tool. Today OAEs are an essential tool in the audiologist's armamentarium.
Audiologists have gained tremendous insight into the putative physiology of OAEs in the last 30 years, and their use has commensurately increased to a wide range of clinical situations (Kemp, 2002). Although many advances have been made during this time, the full scientific and clinical potential of OAEs has yet to be realized. Basic questions about the generation and propagation of OAEs are still being investigated, and uses of OAEs beyond a tool to aid in the diagnosis of hearing loss are still being discovered. This paper provides a brief retrospective of the physiological origin of OAEs as revealed in the early days of OAE research, and discusses issues related to OAEs that are debated in the literature today. Finally, a series of case studies is presented where OAEs were clinically useful although not necessarily in their traditional role as a hearing screening tool.
A Brief History
In the first published paper on OAEs, Kemp (Kemp, 1978) convincingly argued that the cochlea was likely the source of OAEs. Kemp demonstrated that he could only record the ringing of a speaker in a metal cavity followed by random noise after playing a series of clicks into the cavity. However, the same stimulus played into a healthy ear produced a response well beyond 10 ms. This was evidence that the response recorded from the human ear was physiological in origin, however, the anatomical source of these emissions could not be discerned based on these findings. In the same publication, Kemp demonstrated that the delay after which these responses would arrive at the recording microphone was frequency dependent. Responses to low-frequency tone bursts exhibited a shorter delay than responses to high-frequency tone bursts. Kemp argued that this frequency-dependent delay was a direct reflection of the tonotopic organization of the cochlea and hence, the origin of the emissions had to be in the cochlea.
Research subsequent to Kemp's findings showed the outer hair cells (OHCs) as the source of OAEs (Brownell, 1990). This knowledge has been critical to discovering the clinical applications of and expectations from OAEs (Gorga et al., 1994;Shaffer & Dhar, 2006). Especially pertinent to their clinical application, for example, is the fact that OAEs are vulnerable to a variety of agents specifically toxic to the OHCs (McFadden & Mishra, 1993;Davis, Qiu, & Hamernik, 2005). Despite the discovery that OAEs come from the OHCs, however, there are still fundamental questions yet to be answered about the generation and propagation of OAEs.
Where in the OHC?
The discovery that the length of the body of the outer hair cell changes upon stimulation (Brownell, Bader, Bertrand & de Ribaupierre,1985) made OHCs a primary candidate for the mechanism responsible for cochlear amplification and the source of OAEs. However, the exact physiological source of the cochlear amplifier has yet to be established beyond reasonable doubt. Somatic motility and stereociliary processes involved in mechanical-to-electrical transduction are being examined as candidate mechanisms (Dallos, 2008). Even if one assumes that the cochlear amplifier is the source of OAEs, the uncertainty over the physiological source of the amplifier makes it impossible to pinpoint the source of OAEs in the OHCs.
The identification of the membrane protein Prestin and its role in somatic motility (Zheng et al., 2000) has opened the door to more direct examination of the role of somatic motility in the generation of OAEs. Investigators have recorded (Cheatham et al., 2004;Liberman et al., 2004), distortion products in the form of OAEs or cochlear microphonics in mice bred without Prestin. This led to the suspicion that OAEs may be generated by nonlinear stereociliary processes. This suspicion appears to be confirmed by the lack of demonstrable OAEs in mice lacking stereocilin, a protein necessary for functional lateral connections between adjacent stereocilia in the hair bundle of the OHC (Verpy et al., 2008). Rapidly evolving techniques and knowledge of genetic manipulations will, in the future, help to unravel the mystery of the exact source of OAEs.
How do OAEs escape the cochlea?
In addition to the puzzle about the source of OAEs, the mode of propagation of OAEs from within the cochlea to the ear canal has not been completely determined. The mode of forward propagation of mechanical energy has traditionally been described using the traveling wave model. As researchers began to examine the minute details about OAEs generation and propagation, the general assumption was that OAEs travel out of the cochlea the same way energy travels into the cochlea, via a traveling wave. This mode of transmission would result in a delay in recording OAEs after initiating a stimulus tone. This delay would be approximately twice the delay for the stimulus to reach its characteristic frequency (CF) place in the cochlea. Evidence is available in support of this mode of OAE propagation (Shera et al., 2002), however, direct measurements of traveling-wave delays in live human ears are not possible. In laboratory animals, where measurements on live subjects are possible, parallel measurements of delays on the basilar membrane and otoacoustic emissions in the ear canal have not shown a clear relationship (Siegel et al., 2005). Efforts to observe backward traveling waves via direct measurements of basilar membrane motion have not been successful (Ren, 2004). In contrast, evidence of backward traveling waves has been reported from measurements of fluid pressure in the cochlear chambers (Dong & Olson, 2008). Thus, the exact mode of propagation of OAEs out of the cochlea and into the ear canal remains unknown at this time.
Clinical use of OAEs
In the computing world, the success of a platform or operating system often depends on the proverbial "killer app", an application that is so necessary and intuitive that it popularizes the whole platform or operating system. Newborn hearing screening was certainly the "killer app" for OAEs in the United States. It is well established that both distortion product OAEs (DPOAEs) and transient evoked OAEs are efficient tools for screening hearing function for all ages (Prieve et al., 1996;Gorga et al., 1997). The performance of DPOAEs in extending this screening function to a more precise prediction of hearing thresholds has led to limited success (Gorga, Neely et al., 1994;Dhar & Shaffer, 2004;Shaffer & Dhar, 2006). Most clinical OAE devices offer the option of utilizing a screening or diagnostic mode, as well as several pre-programmed test protocols. The clinician can also create a "custom" test protocol by choosing the recording frequency range and the density or frequency resolution of the test. With the development of the understanding of the physiology behind OAEs comes the opportunity to exploit them for a wider variety of purposes. As new applications emerge, researchers will need to explore the clinical application of test parameters outside those established for screening hearing loss.
Case Studies
Here, a montage of cases is presented where OAEs have been utilized for a variety of clinical purposes.
Example A: Newborn Hearing Screening Failure, Patients RD & CS
OAEs are frequently used to follow up on infants that have failed a newborn hearing screening. When robust responses are seen, the assumption is that sensory function is good. Two examples of robust OAEs from babies who failed newborn hearing screenings, but passed upon subsequent evaluation are presented in Figure 1. While all tests of hearing function depend on the forward transmission of sound energy through the outer and middle ears into the cochlea, this path has to be traversed twice in the case of OAEs. The first time is when stimuli travels into the cochlea, the second is when the OAEs are transmitted back to the ear canal from the cochlea. OAEs are particularly sensitive to small alterations in outer and middle ear transmission characteristics. Absent OAEs are often caused by residual debris in the newborn's outer ear, not because of a cochlear pathology. Simply allowing this debris to clear out of the outer ear can result in robust OAEs, as seen in the two examples in Figure 1.
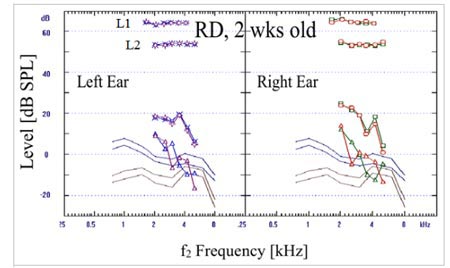
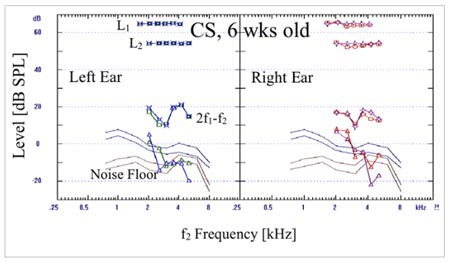
Click Here to View Larger Version of Figure 1 (PDF)
Figure 1. Examples of robust bilateral DPOAEs from two infants: RD = 2 weeks old, and CS = 6 weeks old. The levels of the two stimulus tones are marked as L1 and L2. Two similar traces of 2f1-f2 level in each case demonstrate repeatability. The noise floor is elevated in the lower frequencies but adequate signal-to-noise ratio is maintained.
Example B: Newborn Hearing Screening Failure, Patient NM
While detection of DPOAEs during follow up of a failed newborn hearing screening can help clear suspicion of an auditory pathology, the absence of OAEs play an important role in determining treatment. Results of two DPOAE tests from patient NM are presented in Figure 2. NM had failed newborn hearing screening despite an unremarkable family history and uneventful pregnancy and birth. DPOAEs could not be recorded above the noise floor when NM was 10 days old (top panels, Figure 2). The otolaryngologist diagnosed bilateral otitis media at the 10-day appointment. NM returned for a second follow up after treatment for the otitis media. At that time normal tympanograms were obtained and the otolaryngologist confirmed clear outer and middle ears. However, NM failed the OAE screening again at this visit (lower panels of Figure 2). Further testing was recommended because of the results of the OAE testing. An Auditory Brainstem Response (ABR) test yielded no response to air or bone conducted clicks or tone bursts. Based on the combined findings of the OAE and ABR tests, the parents were advised that the findings were consistent with severe bilateral sensorineural hearing loss. The family is currently under the care of a local cochlear implant program.
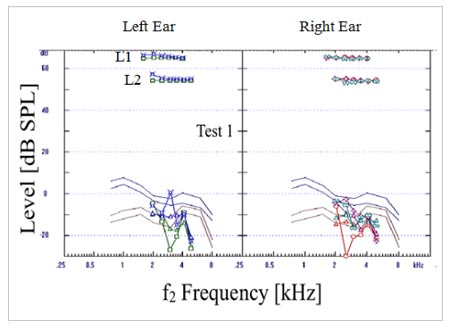
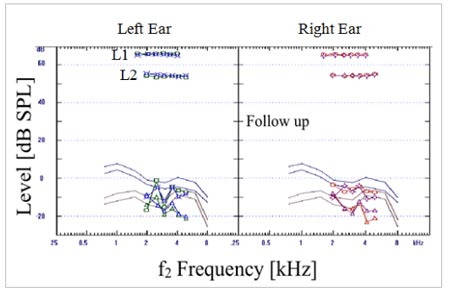
Click Here to View Larger Version of Figure 2 (PDF)
Figure 2. Results of two sets of DPOAE measurements from baby NM. Results in the top two panels represent recordings made when NM was 10-days old. Results from follow up testing approximately one month later are displayed in the lower two panels. No DPOAEs were recordable significantly above the noise floor in either test session.
Example C: Functional Hearing Loss, Patient SK
Since OAE testing is an electrophysiological measure that does not require a behavioral response from the patient, it is useful in diagnosing a patient who is unable or unwilling to participate in pure tone testing, as well as in ruling out functional hearing loss. SK, a 9-year-old female, presented with the complaint that she could not hear out of her left ear. She failed both a school hearing screening and a hearing screening at her pediatrician's office. Her behavioral hearing thresholds showed essentially normal thresholds in the right ear and a mixed moderate hearing loss in the left ear (Figure 3). Tympanograms were consistent with normal middle ear pressure and function. Middle ear muscle reflexes were absent in the left ear irrespective of whether an ipsilateral or contralateral stimulus was presented. Middle ear muscle reflexes were recorded for ipsilateral and contralateral stimulation at 100 dB SPL when the probe was in the right ear. Robust OAE's were recorded from both ears (Figure 4). Note that the continuous lines without symbols represent the range of DPOAE levels between the 5th percentile of a normal-hearing group (lowest line) and 95th percentile of an impaired group (highest line). Recorded DPOAE levels from both of SK's ears exceed the upper bounds of the range (Gorga et al., 1997) and DPOAE levels are comparable between the two ears. ABR to click stimuli showed symmetrical latencies recorded within normal limits with good wave morphology. Responses to click stimuli were observed down to 15dB nHL bilaterally. The symmetry in OAE responses between the two ears provided evidence that the conductive mechanism and the outer hair cells were functional. The ABR results helped confirm that the inner hair cells and neural system up to the brainstem were functional as well. The combination of robust OAEs and ABR recordings helped confirm functional hearing loss.
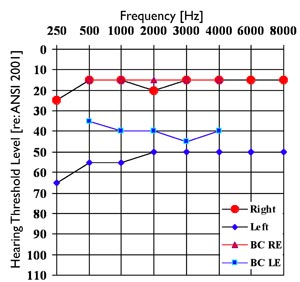
Figure 3. Pure tone audiogram of SK, 9-yr old female, indicating normal hearing sensitivity in the right ear and a moderate mixed hearing loss in the left ear.
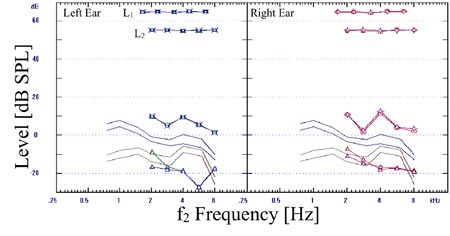
Click Here to View Larger Version of Figure 4 (PDF)
Figure 4. DPOAE recordings from SK (9-yr old female). The display follows the same convention as in Figures 1 and 2. The four continuous lines without symbols mark the normative range of DPOAE levels from Gorga et al. (1997).
Example D: Tinnitus and High Frequency Asymmetry, Patient JD
There is increasing recognition that OAEs can provide indications of cochlear dysfunction when less sensitive measures, such as the pure tone audiogram, show results within normal limits. This is especially true in patients with tinnitus (Hall, 2000). JD, a 48-year-old male, complained of bilateral tinnitus, worse on the right side. The patient's medical history was unremarkable, with no history of noise exposure or vertigo. Pure tone hearing thresholds measured up to 12,500 Hz are displayed in Figure 5.
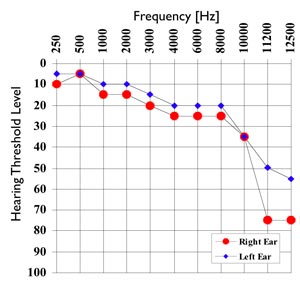
Figure 5. Pure tone hearing thresholds measured to 12,500 Hz from subject JD. Hearing thresholds are within 5 dB across the two ears to 10,000 Hz. Thresholds from the right ear are significantly elevated compared to the left ear at higher frequencies.
Pure tone thresholds were symmetrical to 10,000 Hz. Thresholds from the right ear are significantly worse than the left ear above 10,000 Hz. The difference between ears is more magnified in the DPOAEs (Figure 6). Bands of reduced DPOAE level are observed in the left ear around 2000 and 4000 Hz. DPOAE levels drop to the noise floor around 8000 Hz in the left ear. In contrast, DPOAEs drop to the noise floor around 5000 Hz in the right ear and a broader band of reduced DPOAE levels is observed between 2000 and 4000 Hz. In this case, significant differences between ears were only observed above 10,000 Hz when measuring pure tone thresholds. DPOAE levels reveal differences between the two ears at lower frequencies. DPOAE levels drop to the noise floor starting at a lower frequency in the right ear, where JD reported the tinnitus being worse, than the left ear. DPOAEs appear to highlight differences between the two ears in a more sensitive manner than pure tone thresholds. Although the relationship between OAEs and tinnitus are just beginning to be explored, cases such as JD show promise for the emerging use of OAEs in these instances.
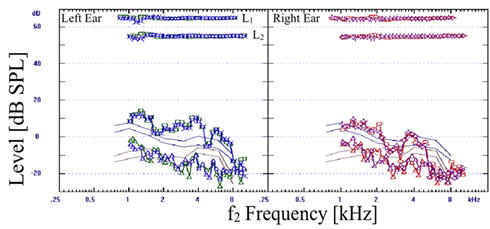
Click Here to View Larger Version of Figure 6 (PDF)
Figure 6. DPOAEs recorded from subject JD. Frequency bands of reduced DPOAEs are noticed around 2 and 5 kHz in the left ear. A broader band of reduced DPOAE levels between 2 and 4 kHz is observed in the right ear. The DPOAEs drop to the noise floor above 4 kHz in the right ear.
Example E: Acoustic Trauma, Patient EC
EC, a 27-year-old male, was seen after being exposed to a piercing fire alarm in a hotel. Subsequent to this acoustic trauma, he reported a plugged feeling in his right ear and postulated that that his left ear was relatively protected as it was against a pillow at the time of the event. EC's medical history was unremarkable with no complaints of tinnitus, vertigo, or otalgia. Pure tone audiometery revealed hearing thresholds well within normal limits bilaterally (Figure 7). However, distinct notches in DPOAE level were observed in repeated recordings in both ears (Figure 8). Upon inquiry, EC reported frequently listening to loud music with earphones. The notches in DPOAE level could be noise induced due to his music listening habits. Note, that this sign of pathology was not discernible in the pure tone audiogram.
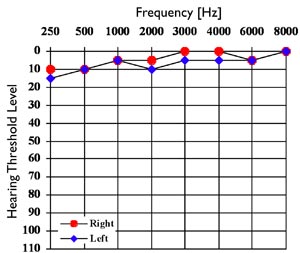
Figure 7. Pure tone hearing thresholds from subject EC (27-year-old male).
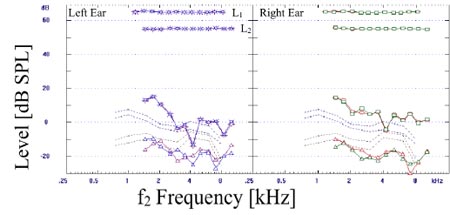
Click Here to View Larger Version of Figure 8 (PDF)
Figure 8. DPOAEs recorded from subject EC. Repeatable notches in DPOAE level are observed in both ears around 4000 Hz.
Example F: Ototoxic Drug Use, Patient CT
CT is a 48-year-old woman being treated for lung cancer. She developed tinnitus in the month of April immediately following chemotherapy treatment of cisplatinum (the month of May is used as marker of timeline in this case, the year is irrelevant). Her initial audiogram in May revealed essentially normal hearing thresholds from 250 Hz through 2000 Hz with a gradually sloping hearing loss beyond (Figure 9).
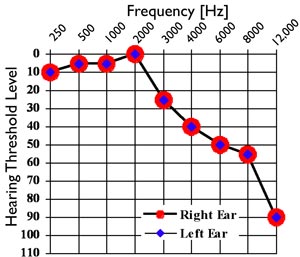
Figure 9. Pure tone hearing thresholds from subject CT (48-yr-old female). Hearing thresholds from initial evaluation in May are displayed but did not show any change during two visits in October of the same year.
A baseline audiogram had not been obtained, making it impossible to determine whether the observed high frequency hearing loss was due to chemotherapy-induced ototoxicity. When pure tone testing was repeated in October of the same year, no significant changes to hearing threshold levels were noted.
DPOAEs were recorded at the first appointment in May, and the results are displayed in the top panels of Figure 10. DPOAE levels show a sharp drop after f2 frequency of 5000 Hz. As was the case with the pure tone audiogram, a baseline measure was not available, and the lack of DPOAEs at frequencies above 5000 Hz cannot be attributed with certainty to chemotherapy. CT was scheduled to receive another chemotherapy treatment during the week following her May visit. She was asked to return for follow up but did not return for the next hearing evaluation until October. The next visit, on October 12, was 2 days post chemotherapy treatment of carboplatinum and VP-16 (Etoposide). Since her first test in May, she did not notice any change in hearing and her tinnitus had remained constant. An audiogram performed at the October 12 visit showed hearing thresholds were unchanged from the previous test in May. DPOAE recordings from October 12 and from two weeks later on October 26 are displayed in Figure 10. While no significant change is observed in DPOAEs recorded between the two dates in October, both of the October recordings show a general reduction in DPOAE level between 2000 and 5000 Hz as compared to the results from May. Recall that
pure tone thresholds did not change between the May and October tests, although a distinct reduction in DPOAE levels was observed during this time frame. Despite the potential evidence of ototoxicity, the exact cause of the changes observed in DPOAE level between the May and October tests cannot be determined with certainty because no baseline testing was performed before the patient began chemotherapy. Cisplatinum and carboplatinum were used for the first and second chemotherapies, respectively. Cisplatinum is known to be toxic to the outer hair cells, while carboplatinum is toxic inner hair cells (Hofstetter, Ding, Powers & Salvi, 1997). Thus the second treatment should have affected inner and not outer hair cells, which are thought to be the source of OAEs. Yet, significant changes in DPOAE levels were observed after the second treatment with carboplatinum. Whether or not the changes in the DPOAE levels were caused by either chemotherapy drug cannot be determined because baselines were not established before each treatment, and monitoring did not occur with regularity through the course of each treatment. If audiologists are to understand and intervene when changes in cochlear function occur due to the administration of ototoxic drugs, timely and serial audiological evaluations including OAE testing must occur.
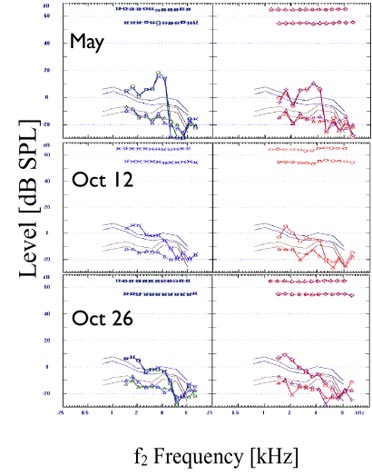
Click Here to View Larger Version of Figure 10 (PDF)
Figure 10. Series of DPOAE measurements from patient CT on three different dates. The first recording, May, was made approximately 4 weeks after cisplatinum therapy. For each date, the left ear data are shown in the left panel in blue, and the right ear data are shown in the right panel in red.
Conclusion
This paper attempts to highlight current understanding of the physiology behind OAEs as well as their clinical usage. While investigators have been successful in answering many questions regarding OAEs, many still remain open and many innovative clinical uses of OAEs are still in their infancy. OAEs will continue to evolve in both the scientific and clinical domains, with new applications for audiologists certain to emerge as well.
References
Brownell, W.E. (1990). Outer hair cell electromotility and otoacoustic emissions. Ear and Hearing, 11, 82-92.
Brownell, W.E., Bader, C.R., Bertrand, D. & de Ribaupierre, Y. (1985). Evoked mechanical responses of isolated cochlear outer hair cells. Science, 227, 194-196.
Cheatham, M.A., Huynh, K.H., Gao, J., Zuo, J. & Dallos, P. (2004). Cochlear function in Prestin knockout mice. Journal of Physiology, 560, 821-830.
Dallos, P. (2008). Cochlear amplification, outer hair cells and prestin. Current Opinion in Neurobiology, 18, 370-376.
Davis, B., Qiu, W. & Hamernik, R.P. (2005). Sensitivity of distortion product otoacoustic emissions in noise-exposed chinchillas. Journal of the American Academy of Audiology, 16, 69-78.
Dhar, S. & Shaffer, L.A. (2004). Effects of a suppressor tone on distortion product otoacoustic emissions fine structure: why a universal suppressor level is not a practical solution to obtaining single-generator DP-grams. Ear and Hearing, 25, 573-585.
Dong, W. & Olson, E.S. (2008). Supporting evidence for reverse cochlear traveling waves. Journal of the Acoustical Society of America, 123, 222-240.
Gorga, M.P., Neely, S.T., Bergman, B.M., Beauchaine, K.L., Kaminski, J.R., Liu, Z. (1994). Towards understanding the limits of distortion product otoacoustic emission measurements. Journal of the Acoustical Society of America, 96, 1494-1500.
Gorga, M.P., Neely, S.T., Ohlrich, B., Hoover, B., Redner, J., Peters, J. (1997). From laboratory to clinic: a large scale study of distortion product otoacoustic emissions in ears with normal hearing and ears with hearing loss. Ear and Hearing, 18, 440-455.
Hall, J. (2000). Handbook of Otoacoustic Emissions. San Diego: Singluar.
Hofstetter, P., Ding, D., Powers, N., & Salvi, R. (1997) Quantitative relationship of carboplatin dose to magnitude of inner and outer hair cell loss and the reduction in distortion product otoacoustic emission amplitude in chinchillas. Hearing Research, 112, 199-215.
Kemp, D.T. (1978). Stimulated acoustic emissions from within the human auditory system. Journal of the Acoustical Society of America, 64, 1386-1391.
Kemp, D.T. (2002). Otoacoustic emissions, their origin in cochlear function, and use. British Medical Bulletin, 63, 223-241.
Liberman, M.C., Zuo, J. & Guinan, J.J., Jr. (2004). Otoacoustic emissions without somatic motility: can stereocilia mechanics drive the mammalian cochlea? Journal of the Acoustical Society of America, 116, 1649-1655.
McFadden, D. & Mishra, R. (1993). On the relation between hearing sensitivity and otoacoustic emissions. Hearing Research, 71, 208-213.
Prieve, B.A., Gorga, M.P. & Neely, S.T. (1996). Click- and tone-burst-evoked otoacoustic emissions in normal-hearing and hearing-impaired ears. Journal of the Acoustical Society of America, 99, 3077-3086.
Ren, T. (2004). Reverse propagation of sound in the gerbil cochlea. Nature Neuroscience, 7, 333-334.
Shaffer, L.A. & Dhar, S. (2006). DPOAE component estimates and their relationship to hearing thresholds. Journal of the American Academy of Audiology, 17, 279-292.
Shera, C.A., Guinan, J.J., Jr. & Oxenham, A.J. (2002). Revised estimates of human cochlear tuning from otoacoustic and behavioral measurements. Proceedings of the National Academy of Sciences of the United States of America, 99, 3318-3323.
Siegel, J.H., Cerka, A.J., Recio-Spinoso, A., Temchin, A.N., van Dijk, P., Ruggero, M.A. (2005). Delays of stimulus-frequency otoacoustic emissions and cochlear vibrations contradict the theory of coherent reflection filtering. Journal of the Acoustical Society of America, 118, 2434-2443.
Verpy, E., Weil, D., Leibovici, M., Goodyear, R.J., Hamard, G., Houdin, C. (2008). Stereocilin-deficient mice reveal the origin of cochlear waveform distortions. Nature, 456, 255-258.
Zheng, J., Shen, W., He, D.Z., Long, K.B., Madison, L.D., Dallos, P. (2000). Prestin is the motor protein of cochlear outer hair cells. Nature, 405, 149-155.

