An otoacoustic emission (OAE) is a low-level sound emitted by the cochlea either spontaneously or evoked by an auditory stimulus. Specifically, OAEs provide information related to the function of the outer hair cells (OHC) (Stach, 2003). Over the past 20 years, their use in routine audiological assessments has increased significantly. Today, OAEs are used commonly in the audiological assessment of difficult to test patients, such as persons who cannot or will not volunteer reliable behavioral responses. OAEs are routinely used in the pediatric population to verify behavioral responses and obtain additional frequency-specific information. In addition, they are routinely used in newborn hearing screening programs across the world. OAEs have many benefits: they are easy to obtain, non-invasive, and provide reliable information regarding cochlear status in a relatively short time.
Present OAEs in an ear indicate many things about the auditory system. First, a present OAE tells us that the conductive mechanism of the ear is functioning properly. This includes proper forward and reverse transmission, no blockage of the external auditory canal, normal tympanic membrane movement, and a functioning impedance matching system. Present OAEs also indicate that OHC function is normal, which, in most cases, correlates with normal hearing sensitivity. OAE testing does have some limitations. OAE testing does not evaluate the inner hair cells (IHC), nVIII, ascending central auditory pathway, or auditory processing function.
A strong knowledge of cochlear anatomy and physiology must exist to understand OAEs. Generally speaking, OAEs are waves generated by movement of the basilar membrane and are measured in the external auditory canal. However, with an in-depth understanding of cochlear anatomy and physiology, OAEs can be directly related to OHC function. There are many events leading up to this. First, there is a stimulus delivered to the ear. This stimulus invokes movement of the basilar membrane, which in turn causes the OHCs to move, or be deflected. When the OHCs move, their stereocilia bend in one direction or the other. Ions rush in and rush out, changing the membrane potential within the hair cell. The changes in voltage across the plasma membrane lead to OHC length changes (shortening and lengthening), which are called electromotility. The electromotility of the OHCs has a feedback effect on the basilar membrane, causing it to vibrate. Therefore, the electromotility of the OHCs is thought to be the mechanism which underlies OAEs. Furthermore, research studies over the past 25 years have demonstrated that when the OHC electromotility is blocked, OAEs are absent (Shehata, et al., 1991; Brownell, 1990), which solidifies the relationship between OHC motility and OAEs.
In addition to vibrating the basilar membrane, the motility of the OHCs causes an amplification of the signal, which is then passed to the IHCs. In turn, the IHCs send a signal to the brain and we then "hear." The OHC motility allows us to be more sensitive to softer sounds. This is called active processing within the cochlea. Without the amplification provided by the OHCs, the IHCs would only be triggered by relatively loud sounds. This is because loud sounds result in larger movements of the basilar membrane, and the IHCs are stimulated directly with little contribution or amplification from the OHCs. This is called passive processing.
One type of OAEs, distortion product OAEs (DPOAEs), studies passive versus active processing to identify auditory function. The passive processing of the cochlea is activated with a stimulus of 70 dB SPL or higher. If a DPOAE is present with this high stimulus level, we know that some structures within the cochlea are responding (or we would not have a response). We cannot, however, conclude it is the OHCs. In fact, it is not the actual movement of the OHCs that is causing the response; likely, it is the vibration of the basilar membrane. Present DPOAEs with a stimulus of 70 dB SPL or greater can only tell us the patient has no greater than a moderate hearing loss. With high stimulus levels we may not be measuring the actual motility (lengthening and shortening) of the OHCs. Instead, the passive response caused by excessive vibration of the basilar membrane may lead one to interpret the response as representative of a normal functioning cochlea, when in fact it is not. With lower intensity levels (commonly used today are L1=65, L2=55), we know we are only triggering the OHCs, the active processes, within the cochlea. If DPOAEs are present with 65 and 55 dB SPL, we can assume normal OHC function and, indirectly, normal hearing.
Transient evoked OAEs (TEOAEs) are a second type of OAE. TEOAEs are stimulated by transient clicks and are often faster and more tolerant to noise and movement than DPOAEs.
More recent research from Shera, 2004, suggests that "...OAEs appear to arise by at least two fundamentally different mechanisms within the cochlea: nonlinear distortion and linear reflection." Shera has constructed a new taxonomy for OAEs based upon what he believes to be their mechanisms of generation. His newer and different views on OAEs challenge what is has been the tradition in regards to classification of OAEs. His research has given us and continues to give us unique and fascinating views on OAEs.
Ototoxicity Monitoring
Over the past decade, three main approaches have emerged for monitoring the effects of ototoxic medications: basic audiologic assessment, high frequency audiometry (HFA; 10-18 kHz), and OAEs (AAA Position Statement, 2009). Using OAEs to monitor ototoxic medications is logical. Ototoxic drugs exert their effect on OHC function (although not solely on OHCs), and OAEs are OHC dependent. With ototoxicity, OAEs have been shown to decrease simultaneously with changes in HFA thresholds and before changes appear in the conventional audiometric frequencies (AAA Position Statement, 2009). Although both TEOAEs and DPOAEs can be used to monitor the effects of ototoxic medications, DPOAEs have some distinct advantages over TEOAEs. First, DPOAEs test higher frequencies than TEOAEs, making them more sensitive to the frequency area affected first. Second, DPOAEs can be recorded in the presence of more hearing loss than TEOAEs. Therefore, if a hearing loss already exists, that patient is still able to be monitored (so long as their hearing loss is not too great), which means DPOAEs can monitor more people. Third, using DPOAEs can provide some indication of degree and configuration of the hearing loss (AAA Position Statement, 2009).
During ototoxicity monitoring, the patient should have OAE testing completed at baseline and before each administration of the ototoxic medication. A logical question about using OAEs to monitor for ototoxicity is what constitutes a significant change in OAE amplitude from one test session to another. Although reports vary, there is no agreed upon universal dB SPL amount that indicates a "significant change" from one test session to the next. A change of 2.4 dB was reported as a significant decrease by Stavroulaki et al. (2002). Clinical experience suggests that changes of 3-6 dB SPL from one test session to the next (while all other test parameters are held constant, or an attempt at that is made) are generally accepted as significant and indicate a change in cochlear function.
In addition to the reasons listed above, OAEs are a good clinical choice in monitoring for ototoxicity because they are quick, which is important for testing children as well as a population who may not feel well due to therapies. In addition, OAEs are cost efficient. Because they can show a change in cochlear function before it appears on the audiogram, further testing can be avoided unless OAE testing suggests a need. The biggest limitation to OAEs for this population is that they are very sensitive to middle ear dysfunction, which is common in children and in those who are immuno-compromised. Although OAEs are being employed for ototoxicity monitoring, they are rarely used in isolation. A change in OAEs from one test session to the next is a strong indicator for the need for more conventional and HFA testing.
Using OAEs in Differential Diagnoses
The use of OAEs to assist in the diagnosis of retrocochlear pathologies has become standard in clinical practice. OAEs arise from the peripheral auditory system; therefore, a logical conclusion is that they will be present in cases of retrocochlear pathology. In most cases this is true; however, neoplasms in the internal auditory canal and/or posterior fossa may impinge on the internal auditory artery and compromise blood flow to the cochlea. This will affect the presence of OAEs. Among various studies, the proportion of patients with retrocochlear pathology showing normal OAEs is about 20% (Hall, 2000). Probably the most common use of OAEs in the diagnosis of retrocochlear pathologies is in the diagnosis of auditory neuropathy (also called auditory dysynchrony or auditory neuropathy spectrum disorder (ANSD)). ANSD is characterized by absent or severely abnormal auditory brainstem responses, poor word recognition, variable audiogram findings, possibly present OAEs, absent middle ear muscle reflexes, and a "mirror image" cochlear microphonic with a change in stimulus polarity. The latter is the gold standard for ANSD, as relying upon an abnormal or absent ABR and present OAEs to diagnose ANSD is not reliable; OAEs may be initially present but disappear over time in individuals with ANSD (Starr et al., 2001, Deltenre et al., 1999).
OAEs and Meniere's Disease
The use of OAEs in the assessment of patients with Meniere's disease has been well documented. Patients with Meniere's disease can essentially be divided into four categories. Van Hufflen et al. (1998) reported that in patients with little hearing loss, OAEs are present, which is expected. When patients have pure-tone thresholds greater than 60 dB HL, OAEs are absent, which is again expected. In patients with Meniere's disease who have hearing thresholds in the intermediate range (30 to 60 dB HL), two categories of patients emerge: patients with relatively large OAEs and patients without measurable emissions. It is this intermediate group that warrants further discussion. In patients with hearing loss of 25-30 dB or greater, OAEs should clearly be absent. However, several studies and authors have recorded OAEs with normal or even greater than expected amplitude values, even with thresholds exceeding 30 dB HL (van Hufflen et al., 1998). It has been hypothesized that these different patterns of OAEs in patients with Meniere's may be a reflection of more than one specific site of lesion (Hall, 2000). It is possible that for these patients with audiometric hearing losses greater than 30 dB HL and present OAEs that the audiometric data is not reflecting OHC activity. Rather, the presence of OAEs in these patients suggests that the OHCs have been spared and the poor hearing thresholds are IHC dysfunction or a disruption at the level of the afferent synapses to IHC and OHC (Hall, 2000). Another possibility to explain this phenomenon is that these findings may be a reflection of the various stages in the pathophysiological mechanism involved in Meniere's disease (van Hufflen et al., 1998). In other words, the damage from the Meniere's has not yet reached the OHCs. Van Hufflen et al. also demonstrated in their 1998 study that OAEs in the contralateral ear with normal hearing in patients with Meniere's disease had smaller OAE amplitudes than persons with normal hearing. They hypothesized that this could indicate an early manifestation of bilateral Meniere's disease.
OAEs in Patients with Tinnitus
Tinnitus has been theorized to originate in both the cochlea (LePage, 1995) and the central auditory system (Reyes et al., 2002). In select patients, the frequency of perceived tinnitus can coincide with a patient's recorded spontaneous OAE (SOAE) frequencies; however, this is not true for everyone, and generally speaking, the relationship between tinnitus and SOAEs has not been found statistically significant (Ceranic et al., 1998). The relationships between tinnitus and DPOAE and TEOAE are even less clear. Ceranic and colleagues (1998) reported that in patients with tinnitus, OAEs are not easily detectable or are abnormal at the tinnitus frequency region, even in patients with normal hearing. Another study reported that in comparison to otologically normal subjects (patients without tinnitus), DPOAE amplitudes were consistently reduced among tinnitus patients, even if the patient had audiometrically normal hearing. The decrement in DPOAEs among tinnitus patients was most pronounced in the 4000-7000 Hz region (Shiomi et al., 1997); however, this is not always the case. Rosanowski and colleagues (1997) divided tinnitus patients into those with hearing loss and those without, and they found no consistent TEOAE results between the two groups. With conflicting results, it is not prudent to conclude that OAEs give objective evidence of tinnitus; more research in this area is needed. Perhaps the most promising area for the use of OAEs with patients with tinnitus is in the area of tinnitus monitoring. Recording OAEs before, during, and after tinnitus retraining therapy may show objective improvements in addition to subjective reports.
Miscellaneous Topics Related to OAEs
The Gorgagram.
Although not an official name, this graph is used by many to plot absolute DPOAE amplitude to obtain information regarding audiometric thresholds. It was derived from a study by Gorga and colleagues (1997), which reported, "...it appears that DPOAE measurements can be used to accurately identify auditory status. An approach is described, using the present data set, that allows one to assign to any measured DPOAE value (DPOAE amplitudes, DPOAE noise) the probability that the response is coming either from the distribution of normal or impaired responses." Depending upon the amplitude of the response, hearing can be determined to fall into the normal range, the abnormal range, or a borderline range (which is different for each frequency tested). The Gorgagram can only be used with DPOAEs, because it is frequency specific between 750-8000Hz. Certain factors must be considered when utilizing this tool: the equipment being used (it is best to use the same equipment as that used in the study), acquisition factors (such as L1, L2), and the recording environment (data was collected in "typical clinical conditions" (Gorga et al., 1997). Overall, this tool is of great use clinically. An example of an adapted Gorgagram is shown in Figure 1.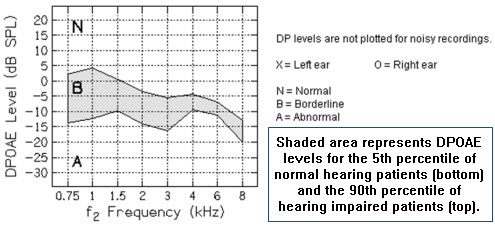
Figure 1. Example of adapted Gorgagram for use in reporting DPOAE test results. Reprinted with permission of AT Still University, Arizona School of Health Sciences, Department of Audiology.
OAEs and hearing aid fittings.
The concept behind this lies in the ability of OAEs to identify regions of the cochlea with damage, which can assist in programming a hearing aid. When OAEs are absent, we assume hearing loss of greater than about 25 dB HL at the frequency where the emission is absent. In difficult to test patients, or any patient for which we cannot obtain audiometric threshold data, the absence of an OAE gives us some idea of hearing levels. In conjunction with ABR, we can use this data to program amplification for these patients. Absent OAEs in conjunction with audiometric thresholds of 70 dB HL or greater can be an indicator of a cochlear dead region, which in turn can influence the hearing aid selection and programming.
OAEs as acoustic fingerprints.
Research at the University of Southampton in the UK (Swabey, Beeby, Brown & Chad, 2004) has reported that OAEs can be used as biometric technology, like fingerprints, to identify individuals. Although OAEs in the cochlea may all be alike, each person's unique middle ear system and external ear change the characteristics of the OAE. Thus, an individual's OAE may be used as an "acoustic fingerprint" to unlock that person's phone or iPod!
OAEs and intraoperative monitoring.
Although not a new concept, using OAEs in the operating room is probably not a common practice in many places. However, OAEs can be used to monitor the function of the nVIII in acoustic neuroma resection surgery. They can also be used to assess hearing in the operating room following grommet tube insertion; however, their absence in these situations may be due to reasons not related to cochlear function (such as edema, blood, mucosa, etc. in the middle or outer ear). When using OAEs for any reason in the operating room, keep in mind that room noise and electrical artifact could interfere with collection.
OAEs in musicians.
Much like the use of OAEs in ototoxicity monitoring, OAEs can be used to provide objective confirmation of cochlear dysfunction in patients with normal audiograms. Similarities between the hearing losses in musicians and industrial workers confirm that excessive exposure to music can affect the ear as much as industrial noise exposure (Hall, 2000). OAE findings can be associated with cochlear frequency specificity; therefore, difficulty hearing can be confirmed with OAEs, even in the presence of a normal audiogram. OAEs can provide an early and reliable warning sign of cochlear dysfunction due to noise/music exposure before any problem is evident on the audiogram. For music professionals, maintenance of hearing not only improves quality of life, but it can preserve their employment and livelihood.
Conclusion
A thorough understanding of OAEs and their anatomical and physiological origin is critical for clinical decision-making. Although OAEs continue to play an important role in routine audiological assessments, they have many applications that are less commonly known. In addition, they hold promise for use in several areas never conceived of 10 years ago. It is exciting to think of what may be ahead for the use of OAEs.
References
American Academy of Audiology (2009). Position statement and clinical practice guidelines: Ototoxicity monitoring. Available from www.audiology.org/resources/documentlibrary/Documents/OtoMonPositionGuideline.pdf
Brownell, W.E. (1990). Outer hair cell electromotility and otoacoustic emissions. Ear & Hearing, 11(2), 82-92.
Ceranic, B.J., Prasher, D.K., & Luxon, L.M.,(1998) Presence of tinnitus indicated by variable spontaneous otoacoustic emissions. Audiol Neurootol, 3(5), 332-44.
Deltenre, P., Mansbach, A.L., Bozet, C., Christiaens, F., Barthelemy, P., Paulissen, D., & Renglet, T. (1999). Auditory neuropathy with preserved cochlear microphonic and secondary loss of otoacoustic emissions. Audiology, 38(4), 187-195.
Gorga, M. P., Neely, S. T., Ohlrich, B., Hoover, B., & Redner, J. (1997). From laboratory to clinic: A large scale study of distortion product otoacoustic emissions in ears with normal hearing and ears with hearing loss. Ear & Hearing, 18, 440-455.
Hall, J. (2000). Handbook of otoacoustic emissions. New York: Singular Publishing Group Thomson Learning.
LePage, E. L. (1995). A model for cochlear origin of subjective tinnitus: Excitatory drift in the operating point of inner hair cells. In: J. Vernon & A. Møller (Eds.), Mechanisms of tinnitus (pp. 115-148). Boston: Allyn and Bacon.
Reyes, S.A., Salvi, R.J., Burkard, R.F., Coad, M.L., Wack, D.S., Galantowicz, P.J., & Lockwood, A.H. (2002) Brain imaging of the effects of lidocaine on tinnitus. Hearing Research, 171(1-2), 43-50.
Rosanowski, F., Hoppe, U., Proschel, U., & Eysholdt, U. (1997). Chronic tinnitus in children and adolescents. HNO, 45(11), 927-32.
Shehata, W.E., Brownell, W.E., & Dieler, R. (1991) Effects of salicylate on shape, electromotility and membrane characteristics of isolated outer hair cells from guinea pig cochlea. Acta Oto-laryngologica, 111(3), 707-718.
Shera, C.A. 2004. Mechanisms of mammalian otoacoustic emission and their implications for the clinical utility of otoacoustic emissions. Ear and hearing; 25(2): 86-97
Shiomi, Y., Tsuji, J., Naito, Y., Fujiki, N. & Yamamoto, N. (1997). Characteristics of DPOAE audiogram in tinnitus patients. Hearing Research 108, 83-88.
Stach, B. (2003). Comprehensive dictionary of audiology illustrated 2nd Ed. New York: Thomson Delmar Learning.
Starr, A., Sininger, Y.S., Nguyen, T., Michalewski, H.J., Oba, S., & Abdala, C. (2001). Cochlear receptor (microphonic and summating potentials, otoacoustic emissions, and auditory pathway (auditory brain stem potentials) activity in auditory neuropathy. Ear & Hearing, 11(3), 215-230.
Stavroulaki, P., Vossinakis, I.C., Dinopoulou, D., Doudounakis, S., Adamopoulos, G. &, Apostolopoulos, N. (2002). Otoacoustic emissions for monitoring aminoglycoside-induced ototoxicity in children with cystic fibrosis. Arch Otolaryngol Head Neck Surg, 128,150-155.
Swabey, M.A., Beeby, S. P., Brown, A.D., Chad, J. (2004). Using otoacoustic emissions as a biometric.
Proceedings of the First International Conference on Biometric Authentication (ICBA 2004), Hong Kong, 600-606.
van Huffelen, W.M., Mateijsen, N.J.M., & Wit, H.P. (1998). Classification of patients with Meniere's disease using otoacoustic emissions. Audiology & Neuro-Otology, 3, 419-430.
Otoacoustic Emissions: Beyond Newborn Hearing Screening
March 14, 2011
Related Courses
1
https://www.audiologyonline.com/audiology-ceus/course/semicircular-canal-dehiscence-40177
Semicircular Canal Dehiscence: Lessons for Collaboration, in partnership with the American Academy of Audiology
This presentation focuses on the audiologic, genetic, radiologic, vestibular, and surgical results of a 10-year-old girl with previously unidentified SSCD. Interprofessional collaboration and how to best assist these children in and out of the classroom with their hearing, technology, and symptoms of the dehiscence will be emphasized.
auditory, textual, visual
129
USD
Subscription
Unlimited COURSE Access for $129/year
OnlineOnly
AudiologyOnline
www.audiologyonline.com
Semicircular Canal Dehiscence: Lessons for Collaboration, in partnership with the American Academy of Audiology
This presentation focuses on the audiologic, genetic, radiologic, vestibular, and surgical results of a 10-year-old girl with previously unidentified SSCD. Interprofessional collaboration and how to best assist these children in and out of the classroom with their hearing, technology, and symptoms of the dehiscence will be emphasized.
40177
Online
PT60M
Semicircular Canal Dehiscence: Lessons for Collaboration, in partnership with the American Academy of Audiology
Presented by Rebekah F. Cunningham, PhD, Devin L. McCaslin, PhD
Course: #40177Level: Advanced1 Hour
AAA/0.1 Advanced; ACAud inc HAASA/1.0; AHIP/1.0; ASHA/0.1 Advanced, Professional; BAA/1.0; CAA/1.0; Calif. SLPAB/1.0; IACET/0.1; IHS/1.0; Kansas, LTS-S0035/1.0; NZAS/1.0; SAC/1.0
This presentation focuses on the audiologic, genetic, radiologic, vestibular, and surgical results of a 10-year-old girl with previously unidentified SSCD. Interprofessional collaboration and how to best assist these children in and out of the classroom with their hearing, technology, and symptoms of the dehiscence will be emphasized.
2
https://www.audiologyonline.com/audiology-ceus/course/innovation-in-hearing-healthcare-presented-37216
Innovation in Hearing Healthcare, presented in partnership with the National Acoustic Laboratories
Build your knowledge with this four-course series presented in partnership with the National Acoustic Laboratories (NAL). A world leader in hearing research and innovation, NAL shares applied research outcomes that are practical to a wide range of expertise, from clinical appointments to technology innovation. Maximize your skills and incorporate evidence-based research in your practice with these short, high-impact webinars designed to improve hearing healthcare.
auditory, textual, visual
129
USD
Subscription
Unlimited COURSE Access for $129/year
OnlineOnly
AudiologyOnline
www.audiologyonline.com
Innovation in Hearing Healthcare, presented in partnership with the National Acoustic Laboratories
Build your knowledge with this four-course series presented in partnership with the National Acoustic Laboratories (NAL). A world leader in hearing research and innovation, NAL shares applied research outcomes that are practical to a wide range of expertise, from clinical appointments to technology innovation. Maximize your skills and incorporate evidence-based research in your practice with these short, high-impact webinars designed to improve hearing healthcare.
37216
Online
PT240M
Innovation in Hearing Healthcare, presented in partnership with the National Acoustic Laboratories
Presented by Brent Edwards, PhD, Vicky Zhang, PhD, Joaquin Tomas Valderrama-Valenzuela, PhD, Simon Alperstein, MSc BE, Paola Incerti, MAudiology, AAudA, Jorge Mejia, PhD, Nicky Chong-White, PhD
Course: #37216Level: Intermediate4 Hours
AAA/0.4 Intermediate; ACAud inc HAASA/4.0; AHIP/4.0; BAA/4.0; CAA/4.0; Calif. SLPAB/4.0; IACET/0.4; IHS/4.0; Kansas, LTS-S0035/4.0; NZAS/3.0; SAC/4.0; Tier 1 (ABA Certificants)/0.4
Build your knowledge with this four-course series presented in partnership with the National Acoustic Laboratories (NAL). A world leader in hearing research and innovation, NAL shares applied research outcomes that are practical to a wide range of expertise, from clinical appointments to technology innovation. Maximize your skills and incorporate evidence-based research in your practice with these short, high-impact webinars designed to improve hearing healthcare.
3
https://www.audiologyonline.com/audiology-ceus/course/20q-update-on-cochlear-implants-38753
20Q: Update on Cochlear Implants: Hearing Preservation, Single-Sided Deafness, and Personalized Fitting
The course reviews new candidacy criteria for cochlear implantation, including when to consider referring for a cochlear implantation evaluation, considerations specific to new patient populations, and outcomes of cochlear implant use observed for these patient populations. The course also reviews the use of imaging to personalize the mapping of cochlear implant and electric-acoustic stimulation devices.
textual, visual
129
USD
Subscription
Unlimited COURSE Access for $129/year
OnlineOnly
AudiologyOnline
www.audiologyonline.com
20Q: Update on Cochlear Implants: Hearing Preservation, Single-Sided Deafness, and Personalized Fitting
The course reviews new candidacy criteria for cochlear implantation, including when to consider referring for a cochlear implantation evaluation, considerations specific to new patient populations, and outcomes of cochlear implant use observed for these patient populations. The course also reviews the use of imaging to personalize the mapping of cochlear implant and electric-acoustic stimulation devices.
38753
Online
PT120M
20Q: Update on Cochlear Implants: Hearing Preservation, Single-Sided Deafness, and Personalized Fitting
Presented by Margaret Dillon, AuD, PhD, CCC-A
Course: #38753Level: Intermediate2 Hours
AAA/0.2 Intermediate; ACAud inc HAASA/2.0; AHIP/2.0; ASHA/0.2 Intermediate, Professional; BAA/2.0; CAA/2.0; Calif. SLPAB/2.0; IACET/0.2; IHS/2.0; Kansas, LTS-S0035/2.0; NZAS/2.0; SAC/2.0
The course reviews new candidacy criteria for cochlear implantation, including when to consider referring for a cochlear implantation evaluation, considerations specific to new patient populations, and outcomes of cochlear implant use observed for these patient populations. The course also reviews the use of imaging to personalize the mapping of cochlear implant and electric-acoustic stimulation devices.
4
https://www.audiologyonline.com/audiology-ceus/course/guidelines-for-determining-ci-candidacy-37498
Guidelines for Determining CI Candidacy, presented in partnership with the ACIA
Considerable variation exists across the medical and audiologic communities regarding determination of cochlear implant (CI) candidacy. While criteria exist from the FDA for children and adults and from CMS for Medicare beneficiaries, different clinics and even audiologists within the same clinic use varying protocols to determine CI candidacy in different age groups and also considering factors other than hearing status. To provide guidance on this topic, the ACI Alliance Board of Directors commissioned four papers to provide guidelines for candidacy for children and adults with bilateral hearing loss and those with single-sided deafness. Task forces were appointed to develop the guidelines with membership drawn from across the care continuum to include audiologists, surgeons, speech-language pathologists, and others involved in CI patient care. This course will review each of those four candidacy guidelines.
auditory, textual, visual
129
USD
Subscription
Unlimited COURSE Access for $129/year
OnlineOnly
AudiologyOnline
www.audiologyonline.com
Guidelines for Determining CI Candidacy, presented in partnership with the ACIA
Considerable variation exists across the medical and audiologic communities regarding determination of cochlear implant (CI) candidacy. While criteria exist from the FDA for children and adults and from CMS for Medicare beneficiaries, different clinics and even audiologists within the same clinic use varying protocols to determine CI candidacy in different age groups and also considering factors other than hearing status. To provide guidance on this topic, the ACI Alliance Board of Directors commissioned four papers to provide guidelines for candidacy for children and adults with bilateral hearing loss and those with single-sided deafness. Task forces were appointed to develop the guidelines with membership drawn from across the care continuum to include audiologists, surgeons, speech-language pathologists, and others involved in CI patient care. This course will review each of those four candidacy guidelines.
37498
Online
PT240M
Guidelines for Determining CI Candidacy, presented in partnership with the ACIA
Presented by Sandra Prentiss, PhD, CCC-A, Daniel Zeitler, MD, FACS, Donna L. Sorkin, MA, Andrea Warner-Czyz, PhD, Margaret Dillon, AuD, CCC-A, Matthew Carlson, MD, Lisa Park, AuD, CCC-A, Nancy Young, MD
Course: #37498Level: Intermediate4 Hours
AAA/0.4 Intermediate; ACAud inc HAASA/4.0; AHIP/4.0; ASHA/0.4 Intermediate, Professional; BAA/4.0; CAA/4.0; Calif. SLPAB/4.0; IACET/0.4; IHS/4.0; Kansas, LTS-S0035/4.0; NZAS/3.0; SAC/4.0; Tier 1 (ABA Certificants)/0.4
Considerable variation exists across the medical and audiologic communities regarding determination of cochlear implant (CI) candidacy. While criteria exist from the FDA for children and adults and from CMS for Medicare beneficiaries, different clinics and even audiologists within the same clinic use varying protocols to determine CI candidacy in different age groups and also considering factors other than hearing status. To provide guidance on this topic, the ACI Alliance Board of Directors commissioned four papers to provide guidelines for candidacy for children and adults with bilateral hearing loss and those with single-sided deafness. Task forces were appointed to develop the guidelines with membership drawn from across the care continuum to include audiologists, surgeons, speech-language pathologists, and others involved in CI patient care. This course will review each of those four candidacy guidelines.
5
https://www.audiologyonline.com/audiology-ceus/course/common-errors-in-diagnostic-audiology-30162
Common Errors in Diagnostic Audiology: Tips for Improving Efficiency, Accuracy and Outcomes
This practical 3-part course series reviews standard diagnostic audiology procedures and the common errors that can lead to clinical inefficiency and inaccurate results. Easy to implement guidance is provided for cleaning up clinical protocols to ensure that audiological testing yields the highest quality information in a cost-efficient manner for an accurate diagnosis and improved patient outcomes.
auditory, textual, visual
129
USD
Subscription
Unlimited COURSE Access for $129/year
OnlineOnly
AudiologyOnline
www.audiologyonline.com
Common Errors in Diagnostic Audiology: Tips for Improving Efficiency, Accuracy and Outcomes
This practical 3-part course series reviews standard diagnostic audiology procedures and the common errors that can lead to clinical inefficiency and inaccurate results. Easy to implement guidance is provided for cleaning up clinical protocols to ensure that audiological testing yields the highest quality information in a cost-efficient manner for an accurate diagnosis and improved patient outcomes.
30162
Online
PT180M
Common Errors in Diagnostic Audiology: Tips for Improving Efficiency, Accuracy and Outcomes
Presented by James W. Hall III, PhD
Course: #30162Level: Advanced3 Hours
AAA/0.3 Advanced; ACAud inc HAASA/3.0; BAA/3.0; CAA/3.0; Calif. SLPAB/3.0; IACET/0.3; IHS/3.0; Kansas, LTS-S0035/3.0; NZAS/3.0; SAC/3.0; Tier 1 (ABA Certificants)/0.3
This practical 3-part course series reviews standard diagnostic audiology procedures and the common errors that can lead to clinical inefficiency and inaccurate results. Easy to implement guidance is provided for cleaning up clinical protocols to ensure that audiological testing yields the highest quality information in a cost-efficient manner for an accurate diagnosis and improved patient outcomes.


