Learning Outcomes
After this course learners will be able to:
- Identify differences between a CIC and BTE style hearing aids.
- Identify when OTC hearing aids were introduced in the USA by FDA rule.
- Identify differences in APHAB and SSQ12 results for aided and unaided conditions of an OTC style hearing aid.
Introduction
Over-the-Counter (OTC) hearing aids were recently introduced in the United States with the Food and Drug Administration (FDA) final rule on October 17, 20221. This rule was enacted to improve access to hearing aids for the over 44 million people2 with hearing loss in the United States. Since that time, a variety of OTC hearing aids have become available. These devices vary in form factor, technology, and overall performance.
As indicated by the name, an OTC hearing aid is meant to be acquired without the intervention of a Hearing Healthcare Professional (HHP). With prescription hearing aids, which do require the intervention of the HHP, there is an evaluation of the hearing and a hearing aid is prescribed and appropriately fitted to the patient. There is also generally follow-up and support for the patient following the fitting. In the case of the OTC, the consumer provides a self-diagnosis of perceived hearing loss, selects the OTC hearing aids from a retailer of their choice, and then performs a fitting procedure on their own.
An essential part of the selection process is choosing a form factor. The form factor is important as it is one way to address cosmetic appeal and size preference. It can also impact handling of the device. Devices can be Behind-the-Ear (BTE), Receiver-in-the-Canal (RIC), In-the Ear (ITE), or Completely-in-the-canal (CIC) style devices. Each style has various advantages for use and handling, and it is important for the consumer to self-educate on what may be most appropriate.
The CIC form factor differs from the RIC and BTE styles in several ways. First, as the name implies, it is a placed completely in the ear canal. For the OTC application, as there is no hearing professional involved to take ear impressions, this type of fitting typically is not a traditional custom fit CIC, but rather an instant-fit design using various sized silicone eartips that securely attach to the hearing aid. However, like traditional CIC devices, the microphone placement is at the opening of the ear canal and better utilizes the effects of the pinna in directing sound to the microphone. The design potentially results in a deeper receiver placement in the ear canal.
The self-fitting of the selected OTC involves placement of the hearing aids in the ear and a means of finding an amplification profile deemed appropriate to the hearing loss. In some cases, this method of selecting appropriate amplification is simply choosing which one sounds the best from a small, closed set of pre-programmed choices within the device. Another option in some OTC hearing aids is to determine a hearing profile via the hearing aid. This is done via listening to a series of beeps using the assistance of an app, and the system places a person into one of a set of hearing profiles with the goal of providing an appropriate amplification setting. It is also possible, in some cases, to make additional limited adjustments to the amplification settings as needed. Any fitting support for the OTC hearing aid is provided by manufacturer literature included with the device, videos from the manufacturer on their website or embedded in the app, or in some cases, telephone support provided by the manufacturer.
For the user of a hearing aid, the fitting approach and form factor are both critical components for success. The consumer has to take an independent approach to the fitting process. In the case of prescription hearing aids, the HHP is an available resource to support the fitting. In the case of the OTC, resources are generally more limited or may be available through an HHP for an additional cost. Additionally, they must choose the style that is most appropriate for them. While the HHP will take into account may factors when suggesting a style of aid, the user will likely only choose the style based on the look and desired appearance. Both the self-fitting and self-selection of the OTC hearing aid may lead to varied success with the device.
Our interest in this study was to evaluate wearer outcomes. Previous studies have suggested comparable results to prescription hearing aid fittings4,5. These studies have reviewed behind-the-ear (BTE) styles as well as receiver-in-the-canal (RIC) styles. This study focused on a CIC style OTC device. As the outcome of an OTC fitting is dependent on wearer perception, both outcome measures selected were subjective questionnaires to give pre- and post-fitting impressions from the user. The Abbreviated Profile of Hearing Aid Benefit (APHAB)6 and a short form of the Speech, Spatial and Qualities of Hearing scale7, (SSQ12)8, were selected as the performance measures with the OTC devices. These were selected to give a benefit measurement as well as a measurement of sound awareness.
Methods
This study was conducted at the University of South Dakota Speech-Language and Hearing Clinic and received appropriate approval from USD IRB. Participants were twenty-six adults (Female: 8, Male:18) aged 34-82 years (mean age 62 years) with bilateral, sensorineural hearing loss (see Figure 1 for audiometric thresholds). All participants’ hearing loss was within the fitting range of the hearing aid. Four of the participants had some successful experience (less than five years) wearing hearing aids. The remaining 22 participants were first-time hearing aid users.
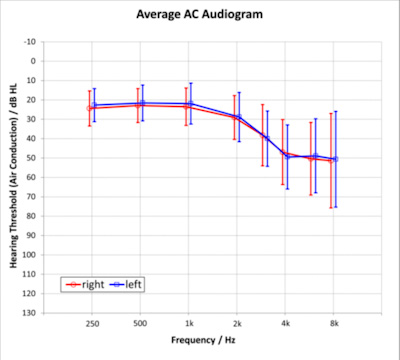
Figure 1. Mean audiometric air conduction thresholds with +/- one standard deviation.
Test Devices
Devices were instant-fit, CIC style hearing aids using vented silicone “Click Sleeves” available in four sizes for coupling to the ear. The devices used 10a zinc air batteries. Wireless capabilities via near field magnetic induction (NFMI) were enabled for communication between devices on each ear. The wireless connectivity between devices provided for alignment in sound environment classification, volume settings, noise reduction characteristics, and some level of directional processing. Devices were able to receive a high-frequency acoustic signal from cell phones which was used to command the hearing aids; however, no wireless or Bluetooth transmission from the hearing aids to any receiving devices was possible.
Device Set-up
Investigators were present in the fitting room with the participants; however, investigators were instructed not to assist the participants in preparing the hearing aids. Participants were provided with written instructions for preparing their devices like the instructions provided within the box of an OTC purchased device. Participants were also provided with a telephone number for fitting support, consistent with real-world phone support for these OTC devices.
All fittings were bilateral. Instructions guided the participants to select and attach the eartip that best fit each ear. Participants were then asked to insert a size 10a battery into each device. Participants were asked to download the fitting app to their mobile phone and pair the hearing aids to the phone via in-app instructions.
Once paired, the fitting app provided instructions for the wearer to determine a hearing profile. Tones were presented via the hearing aids, and participants confirmed audibility or lack thereof via the fitting app. Based on the resulting hearing profile, the devices provided an initial amplification setting. Participants were able to adjust settings to a comfortable level as they deemed appropriate. Further capabilities in the app to adjust gain settings and make minor programming adjustments were available for use during the two-week wearing period.
Test Measures
Participants completed the APHAB questionnaire prior to the fitting of the devices (unaided) and upon completion of the two-week field test (aided). The APHAB provides input across four different subscales. These subscales are ease of communication (EC), background noise (BN), reverberant room (RV) and aversiveness (AV). A global, overall score is also calculated from the three subscales EC, BN and RV. Higher scores in any of the subscales indicate more challenges for the respective subscale. Lower scores represent less hearing difficulties for the respective subscale.
The SSQ12 questionnaire is a short form (12 questions) version of the SSQ (49 questions) which assesses the impact of hearing loss across the characteristic domains of speech, spatial and other qualities of hearing such as effort and naturalness. Scores range from 0 (greatest difficulty) to 10 (no difficulty). As with the APHAB, the SSQ12 was conducted in the unaided and aided (post-wearing test) conditions.
Results
All participants were able to self-fit the hearing aids and proceed with the two-week wearing period.
Figure 2 displays the APHAB results. The mean unaided results for each of the APHAB subcategories of EC, BN, RV, AV and Global were 28.8, 43.8, 37.3, 26, and 36.6, respectively. The mean aided results for the same categories were 15.7, 32.3, 25.6, 31.2, and 24.5. Mean benefit results for each subcategory, again in the same order, were 13.1, 11.5, 11.7, -5.2, and 12.1.
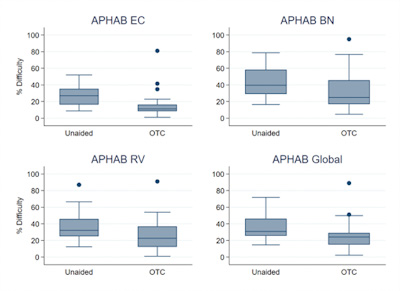
Figure 2. Box plots of APHAB data. The Ease of Communication (EC, top left), Background Noise (BN, top right), Reverberant Room (RV, bottom left) subscales as well as the global score (bottom right) are plotted. The boundaries of the shaded boxes represent the 25th and 75th percentiles. The lines within the boxes indicate the medians. Error bars indicate minimum and maximum values. Filled circles are the outliers.
Unaided and aided APHAB scores were compared using a repeated-measures ANOVA. Results indicated a significant improvement in the subscales EC (F1,25 = 17.17, p < .001), BN (F1,25 = 8.34, p = .01), and RV (F1,25 = 8,62, p = .01) as well as for the global score (F1,25 = 12.63, p < .01). No significant difference was determined for AV (F1,25 = 2.26, p = .15). Positive correlation was identified between degree of hearing loss as measured by 3-frequency pure tone average (PTA) and the EC, RV and Global subscales (ρ = .5, ρ = .46, and ρ = .45, respectively, all p < .05). That is, the more severe the hearing loss, the more benefit participants perceived. No significant correlation was observed between PTA and the BN and AV subscales (ρ =0.34, p =.09).
Mean unaided scores for SSQ categories of speech, spatial, other and overall were 4.4, 6.8, 5.9 and 5.5, respectively. For the aided conditions, in the same order, results were 6.2, 7.2, 7, and 7.6. Mean aided benefit scores were 1.7, 0.4, 1.0, and 1.2, again, respectively for each category. The SSQ results are displayed in Figure 3. A repeated measures ANOVA with indicated a significant difference between aided and unaided conditions for the categories of Speech (F1,25 = 17.58, p < .001), Qualities (F1,25 = 6.87, p = .01), and Overall (F1,25 = 12.91, p < .01). No significant difference was indicated for the Spatial category (F1,25 = 1.17, p = .29). Also, no significant correlation between SSQ results and PTA were observed.
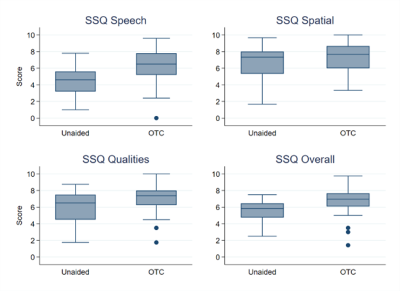
Figure 3. Box plots of SSQ12 data for unaided and aided conditions. The Speech (top left), spatial (top right), qualities (bottom left) domains as well as the overall score (bottom right) are plotted. The boundaries of the shaded boxes represent the 25th and 75th percentiles. The lines within the boxes indicate the medians. Error bars indicate minimum and maximum values. Filled circles are the outliers.
Discussion
In this study, we evaluated the unaided and aided performance of hearing aid wearers using self-fit OTC devices worn completely in the canal (CIC). Measures of both the APHAB and the SSQ12 indicated overall significant improvement with the OTC devices over the unaided condition. Although one would generally expect to see benefits with hearing aids over an unaided condition, there is a possible risk that undesired outcomes may occur without professional intervention. In this study, the outcomes were shown to be quite positive.
With the APHAB results, the three communication subscales, as well as the Global score, all indicated significant benefits over the unaided condition. The strongest result was seen with the EC scale, suggesting that the hearing aids helped improve communication for the hearing aid wearer compared with not wearing any device. Both the RV and BN scores reflect improvement in noisy situations. The OTC devices do provide noise reduction and directional algorithms to help suppress unwanted background noise and these results suggest that these features address background noise concerns and may contribute to the hearing aid wearer’s perception of benefit. These three subscales determine the Global score which also shows a significant improvement over the unaided condition. The AV subscale was the only scale that did not provide significant improvement of the aided condition over the unaided condition. However, this is not surprising as inexperienced hearing aid wearers are likely to notice and find a negative association with new, unwanted background sounds. Previous research has suggested increased AV scores on the APHAB with new hearing aid fittings9. Thus, the lack of a significant change in AV scores shows that the improvement in communication does not come at the cost of an increased aversiveness to the (amplified) sounds.
Additionally, the relationship between PTA and APHAB benefit is somewhat consistent with previous reports. Dornhoffer et al.9 reported a moderate, positive correlation between the RV subscale and PTA but no significant correlations with other subscales. One factor that may influence these results is that, as an OTC fitting, the devices were set to a wearer’s comfort level and not to a prescriptive target based on the audiogram.
The SSQ12 categories of Speech and Qualities as well as the Overall category suggested significant improvement for the aided over the unaided conditions. The one area that did not indicate significant improvement was the spatial category. This is not surprising considering the participants generally had good hearing in the low frequencies, as would be expected of the OTC candidate. The hearing loss in the higher frequencies would have little effect on localization10. The devices used were a CIC style and, therefore, the natural pinna effects for localization were maintained. The use of CIC-style hearing aids has also shown little effect on localization for the degree of hearing loss used in this study10. It can be expected that participants, when wearing hearing aids, experienced little change in localization abilities while finding improvements with speech and other qualities of hearing.
Considering the lack of a correlation between PTA and SSQ12 scores, this may be related to the participants having a mild to moderate hearing loss and, therefore, similar PTAs. A range of patients including higher degrees of hearing loss may show different results, but they would be outside of the fitting range of this device. Ou et al11 reported differences between two groups separated with a 40 dB HL PTA cut-off and did report a significant relationship between SSQ12 scores and PTA groups. Based on PTA, our participants would have only been included in that lower (better) PTA group and, therefore, a significant correlation would not be expected.
The study tools used in this test were subjective test measures provided both before wearing the hearing aids and after two weeks of wearing the hearing aids. Objective test measures may show different results. The measures used to evaluate how the participants performed may not have measured optimal potential with hearing instruments. It can be determined that for wearers with minimal or no experience with amplification, the OTC hearing aid can provide positive perceptions of performance.
Summary
Overall, results clearly suggest that wearers reported significant improvements in their hearing abilities using self-fit CIC style OTC hearing aids. This type of OTC hearing aid is cosmetically appealing due to its small size and placement inside the ear canal. All participants were able to successfully self-fit the hearing aids and reported notable improvements with the devices. These results indicate that this style of hearing aid can provide successful outcomes for those with perceived mild to moderate hearing loss who are interested in OTC hearing aids.
References
Food and Drug Administration, Department of Health and Human Services. (2022). Medical devices; ear, nose, and throat devices; establishing over-the-counter hearing aids. Federal Register, 87(50698), 65.
Goman, A. M., Reed, N. S., & Lin, F. R. (2017). Addressing estimated hearing loss in adults in 2060. JAMA Otolaryngology–Head & Neck Surgery, 143(7), 733-734. https://doi.org/10.1001/jamaoto.2017.0283
Ruusuvuori, J. E., Kukkonen, M. L., Kivelä, T., Hietanen, M., & Levo, H. (2021). Studies on stigma regarding hearing impairment and hearing aid use among adults of working age: A scoping review. Disability and Rehabilitation, 43(3), 436-446. https://doi.org/10.1080/09638288.2019.1630685
De Sousa, K. C., Manchaiah, V., Munro, K. J., Stone, M. A., & Moore, B. C. (2023). Effectiveness of an over-the-counter self-fitting hearing aid compared with an audiologist-fitted hearing aid: A randomized clinical trial. JAMA Otolaryngology–Head & Neck Surgery, 149(6), 522-530. https://doi.org/10.1001/jamaoto.2023.1210
Swanepoel, W., Manchaiah, V., Klyn, N., Hopkins, C., & Moore, B. C. (2023). Comparing hearing aid outcomes in adults using over-the-counter and hearing care professional service delivery models. American Journal of Audiology, 32(2), 314-322. https://doi.org/10.1044/2023_AJA-22-00179
Cox, R. M., & Alexander, G. C. (1995). The abbreviated profile of hearing aid benefit. Ear and Hearing, 16(2), 176-186. https://doi.org/10.1097/00003446-199504000-00005
Gatehouse, S., & Noble, W. (2004). The Speech, Spatial and Qualities of Hearing Scale (SSQ). International Journal of Audiology, 43(2), 85-99. https://doi.org/10.1080/14992020400050014
Noble, W., Jensen, N. S., Naylor, G., Bhullar, N., & Akeroyd, M. A. (2013). A short form of the Speech, Spatial and Qualities of Hearing Scale suitable for clinical use: The SSQ12. International Journal of Audiology, 52(6), 409-412. https://doi.org/10.3109/14992027.2013.781278
Dornhoffer, J. R., Danner, C., Mennemeier, M., Bowen, A., & Lopez, M. (2020). Assessment of hearing aid benefit using patient-reported outcomes and audiologic measures. Audiology & Neurotology, 25(4), 215-223. https://doi.org/10.1159/000507835
Byrne, D., & Noble, W. (1998). Optimizing sound localization with hearing aids. Trends in Amplification, 3(2), 51-73. https://doi.org/10.1177/108471389800300202
Ou, H., & Kim, E. (2017). Which short version of the Speech, Spatial, and Qualities of Hearing Scale to choose: SSQ5 or SSQ12? Journal of Otorhinolaryngology Disorders and Treatments, 1(1).
Citation
Branda, E., Phelan, J., Littmann, V., Lelic, D., & Jorgensen, L. (2025). Subjective wearer results with over-the-counter, self-fitted cic hearing aids. AudiologyOnline, Article 29238. Available at www.audiologyonline.com






