MED-EL EAS Cochlear Implant System Clinical Trial Results and FDA Approval
AudiologyOnline: Tell us about recently receiving FDA approval for the MED-EL EAS Cochlear Implant System.

Allison Racey, AuD: Electric-acoustic stimulation (EAS) combines the electric stimulation of a cochlear implant with the acoustic amplification of a hearing aid for patients with residual hearing in the low frequencies and poor hearing in the high frequencies. Candidates for EAS are adults with normal hearing to moderate sensorineural hearing loss in the low frequencies and severe to profound sensorineural hearing loss in the high frequencies. EAS candidates typically score 60% or less on monosyllabic (CNC) words in quiet in each ear with a hearing aid. MED-EL recently received FDA approval for the EAS System after conducting a clinical trial in the United States with 73 patients implanted with the EAS System. Patients who enrolled in the clinical trial met the same audiologic candidacy criteria as the FDA approval mentioned above.
AudiologyOnline: How are EAS candidates different from traditional cochlear implant candidates?
Allison: Patients who are candidates for EAS are those who may be long-time hearing aid users and have reached a point where acoustic amplification alone is not enough to provide valuable speech information typically found in the high frequencies. These are patients who may frequently complain that they can hear but not understand speech. The potential to lose that residual low-frequency hearing can be a concern for many EAS candidates, but the results from the clinical trial show that a high percentage of subjects maintained residual hearing. This is possible because of some of the unique characteristics of the MED-EL SYNCHRONY EAS System.
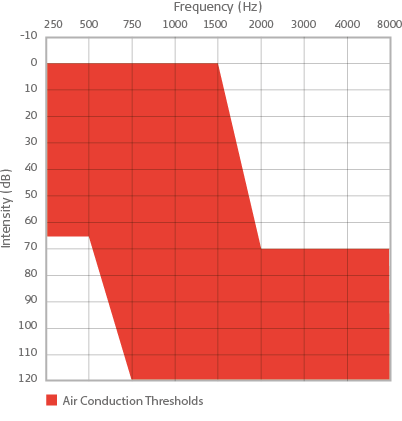
Audiogram showing EAS candidacy.
AudiologyOnline: What are those unique characteristics of the MED-EL SYNCHRONY EAS System?
Allison: The SYNCHRONY EAS System includes an internal cochlear implant and an external processor that provides both electric stimulation and acoustic amplification in one device. The FDA approval for EAS was granted for the SYNCHRONY cochlear implant, which initially received FDA approval in 2014 (for traditional cochlear implant candidates). EAS is approved for use with the SYNCHRONY implant and the FLEX24 electrode array, which is 24 mm in length and utilizes Flex Tip technology for atraumatic electrode insertion. Additionally, the SYNCHRONY is the only cochlear implant currently FDA approved for MRI at 3.0 Tesla with the magnet in place.
AudiologyOnline: What can you tell us about the external processor?
Allison: FDA approval also included the SONNET EAS processor, which is the latest and third-generation of combined EAS technology from MED-EL. This audio processor is based on the already-approved SONNET Audio Processor, with the same features and the addition of acoustic amplification. The SONNET EAS Audio Processor is used with an acoustic ear hook and an earmold, to enable the user to benefit from amplification. Additionally, the SONNET EAS was recently approved for use with the added features of directional microphones and wind noise reduction.

SYNCHRONY EAS Cochlear Implant System.
AudiologyOnline: In the clinical trial, what outcomes were obtained in terms of speech perception?
Allison: The 73 subjects enrolled in the clinical trial performed, on average, 42 percentage points better on sentences in noise with EAS at 12 months post-operatively than they did pre-operatively with hearing aids. The average pre-operative score with hearing aids on sentences in noise was 31% and the average score at 12 months post-operative with EAS was 73%. On monosyllablic words in quiet, subjects demonstrated a 36.5 percentage-point improvement with EAS compared to the pre-operative hearing aid condition. Subjects also showed significant improvement when comparing the combined EAS condition to an electric-stimulation only map at 12 months, demonstrating benefit from adding acoustic amplification.
AudiologyOnline: What did patients report about their performance with EAS?
Allison: Overall, 90% of subjects reported that they experienced an improvement in listening with EAS compared to pre-operatively with hearing aids. Specifically, 92% of subjects reported an improvement in background noise, which is typically an environment in which patients with this type of hearing loss struggle to hear. Out of 73 subjects implanted, 86% reported an increase in satisfaction with EAS compared to their pre-operative hearing aids.
AudiologyOnline: How often did patients lose residual hearing in the clinical trial?
Allison: Residual hearing was used to determine whether or not subjects could be fit with the acoustic amplification (Acoustic Unit) portion of the audio processor. Following implantation, 71 of 73 subjects, or 97%, were able to be fit with the acoustic unit. We also analyzed how much shift subjects experienced in the low frequencies, by comparing the low-frequency pure-tone average (LF-PTA, 250 – 1000 Hz) at the pre-operative interval to the 12-month follow-up. Seventy-nine percent of subjects in the clinical trial experienced less than a 30 dB shift in the LF-PTA. Additionally, out of the 67 subjects who were followed to the 12-month interval, only 12% (8/67 subjects) had a LF-PTA in the profound or total hearing loss range.
AudiologyOnline: So what happens to patients who do end up with a LF-PTA in the profound or total hearing loss range?
Allison: Of the eight subjects in the total/profound hearing loss range, six subjects were still able to be fit with the Acoustic Unit. Five of the six subjects in this group performed better with EAS than they did with electric stimulation alone on at least one of the speech perception tests. Additionally, for the two subjects who were unable to be fit with the Acoustic Unit, performance improved at 12 months with electric stimulation alone compared to preoperatively with hearing aids, by 63 and 78% on CUNY sentences in noise and by 18 and 48% on CNC words in quiet. Overall, none of the eight subjects in the total/profound hearing loss group performed poorer on both speech perception tests post-operatively than they did pre-operatively with hearing aids.
AudiologyOnline: What factors do you think impacted the positive results on residual hearing?
Allison: MED-EL has a strong history of atraumatic design for preservation of existing cochlear structures. The electrode approved for EAS is designed for maximum flexibility so as not to cause unnecessary damage in the cochlea. In addition, surgeons in the clinical trial followed strict guidelines for the EAS surgical technique. These included administering intravenous antibiotics and corticosteroids intra-operatively, eliminating blood and bone dust, applying corticosteroid solution and lubricant at the site of the cochleostomy or round window insertion, changing or washing gloves prior to handling the electrode, and inserting the electrode slowly and gently. Most of the patients in the clinical trial were implanted via a round window approach to inserting the electrode in the cochlea. This combination of factors led to 88% of the subjects in the clinical trial maintaining a low-frequency PTA better than a profound hearing loss.
AudiologyOnline: How can audiologists get more information on EAS?
Allison: Audiologists can contact their local MED-EL representative to find out about EAS trainings in their area. Further information, resources, and CE courses from MED-EL can also be found on the MED-EL Expo on AudiologyOnline, as well as on our website, www.medel.com/us

