Understanding Lindsay-Hemenway Syndrome: Mechanisms, Diagnosis, and Treatment
Introduction
Benign Paroxysmal Positional Vertigo (BPPV) is the most common vestibular disorder, caused by dislodged otoconia entering the semicircular canals (SCs), leading to inappropriate ampullary stimulation by movements like gravity. It results in brief episodes of vertigo and positional nystagmus, which varies based on the affected SC. Different types of BPPV involve different SCs, and specific canalith repositioning maneuvers (CRM) have been designed to effectively treat each type. While most cases are idiopathic, some stem from other inner ear conditions and are classified as secondary BPPV.
Acute unilateral vestibulopathy (AUVP) refers to a sudden loss of the peripheral vestibular function, caused by either a partial or complete lesion on one side. Though the pathomechanisms underlying AUVP are still debated, the most accepted theories involve either a neural insult due to a viral reactivation (vestibular neuritis) or an ischemic lesion of the inner ear. This lesion usually affects the superior division of the vestibular nerve (VN), which innervates the same parts of the inner ear supplied by the anterior vestibular artery, specifically the utricle, the horizontal (HSC) and the anterior SC (ASC).
AudiologyOnline: What is the Lindsay-Hemenway Syndrome?
Andrea Castellucci: Lindsay-Hemenway Syndrome (LHS) is a clinical condition where BPPV follows an ipsilesional AUVP. This syndrome typically develops in two distinct phases.
First phase: patients experience sudden onset vertigo due to AUVP, resulting in an acute, non-positional vertigo accompanied by unilateral vestibular hypofunction, which progressively and slowly improves over time.
Second phase: after a variable period of quiescence, often weeks to months, patients’ symptoms worsen as they develop positional vertigo spells due to a BPPV in the affected ear. This occurs as otoconia dislodge from the damaged utricle and migrate into the ipsilesional posterior SC (PSC), leading to positional vertigo. More rarely, AUVP and BPPV occur simultaneously.
John R. Lindsay and William G. Hemenway were the first to describe a case of AUVP followed by positional vertigo, which is now thought to have been a case of PSC-BPPV. When BPPV occurs as a consequence of AUVP - known as Lindsay-Hemenway syndrome (LHS) - it typically involves the PSC on the same side.
AudiologyOnline: Why BPPV usually involves the PSC in Lindsay-Hemenway Syndrome?
Andrea Castellucci: This is believed to be due to the peculiar anatomy of the inner ear. Since AUVP due to a vestibular neuritis mainly involves the superior VN division, the saccule and PSC, which are innervated by the inferior division, are often left unaffected (Figure 1). Similarly, a selective ischemic lesion of the anterior vestibular artery would damage the same structures sparing the PSC and the saccule (Figure 2). Damage to the utricle can lead to the release of otoconia, which may then settle in the ipsilateral PSC, stimulating the still-functional canal and causing secondary BPPV. The development of PSC-BPPV after AUVP suggests that the PSC remains functionally intact. LHS is not an uncommon condition. According to the literature, a secondary PSC-BPPV occurs in less than 10% of patients with a history of ipsilateral AUVP (either of viral or ischemic origin). LSH has been described even in sequential AUVP sparing the PSC. Nevertheless, a few reports described cases of PSC-BPPV following a complete vestibular lesion and HSC-BPPV following an AUVP.
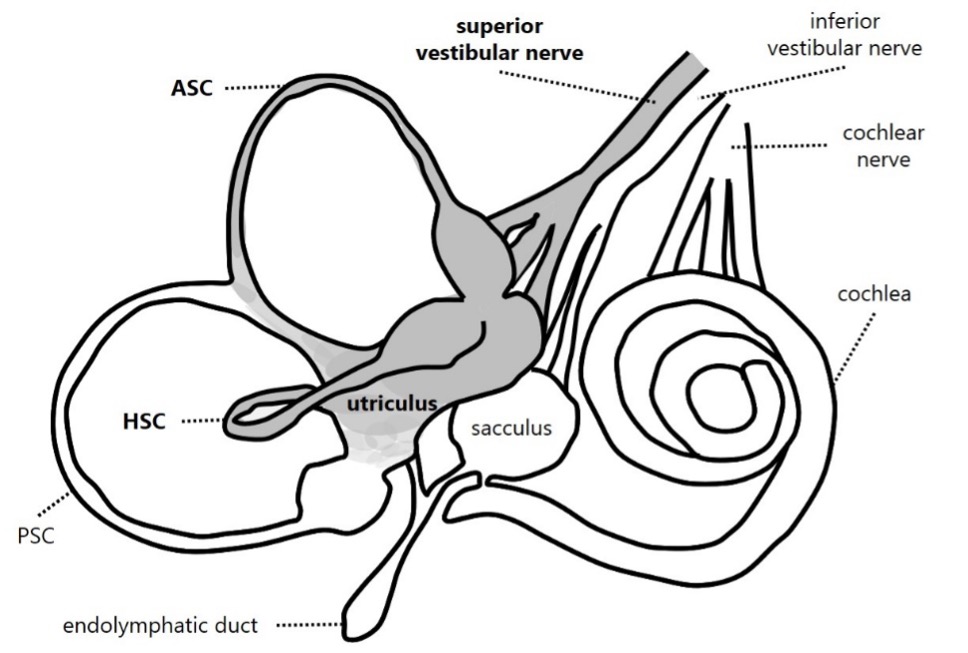
Figure 1. Schematic representation of inner ear innervation showing a vestibular neuritis involving the superior division of the vestibular nerve. Involved receptors and nervous structures are represented in grey and mentioned in bold.
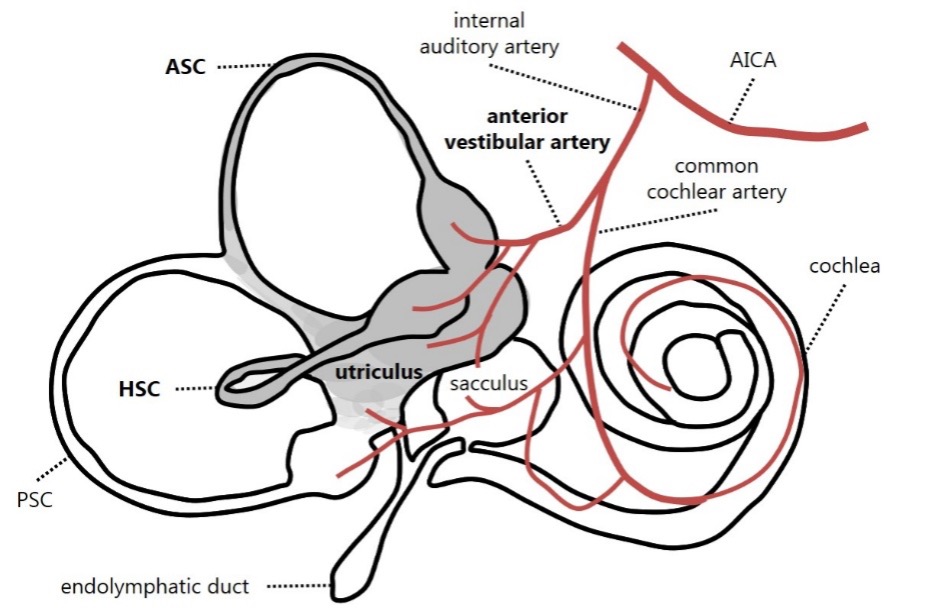
Figure 2. Schematic representation of inner ear vascular supply showing an ischemia involving the labyrinthine structures irrorated by the anterior vestibular artery. Involved receptors and vascular structures are represented in grey and mentioned in bold.
AudiologyOnline: How can we diagnose a Lindsay-Hemenway Syndrome?
Andrea Castellucci: The diagnosis is primarily clinical, based on patient history and symptom progression. Instrumental testing should be given, at least in a second stage, to objectify the functional status of the vestibular receptors / afferents. The bedside assessment, with video-Frenzel goggles, can detected different scenarios depending on the time elapsed from the AUVP and the onset of BPPV
- In case of simultaneous AUVP and BPPV (or in case of early onset BPPV), clinicians can detect a spontaneous paretic nystagmus beating toward the healthy side together with a positive head impulse test (HIT) on the affected side. Either in Dix Hallpike position or in Semont diagnostic test on the affected side, a sudden paroxysmal upbeating ipsi-torsional nystagmus is detected (Video 1).

Video 1. A case of right LHS with simultaneous AUVP and ipsilateral PSC-BPPV. Spontaneous paretic left-beating nystagmus increased by skull vibrations (consistent with right AUVP) is detected with video-Frenzel goggles. Upbeating/right-torsional paroxysmal positional nystagmus (consistent with right PSC-BPPV) is elicited in right Dix Hallpike positioning. After an Epley’s CRM, positional nystagmus recedes leaving the baseline ongoing spontaneous nystagmus.
- In case of late onset BPPV, the patients generally no more exhibit spontaneous nystagmus due to compensation mechanisms, while they still develop paretic nystagmus after head shakings or skull vibrations and a positive HIT on the affected side in case the vestibular function has not fully recovered. In some cases, it is possible to observe a transient upbeating ipsi-torsional nystagmus by bending the head backward, due to a transient ampullofugal shift of otoliths within the involved PSC. Even in this case, both Dix Hallpike and Semont position on the affected side generates a sudden paroxysmal upbeating ipsi-torsional nystagmus (Video 2). In case of complete vestibular restoration of AUVP, patients might only exhibit positional nystagmus with no signs of previous vestibular loss.
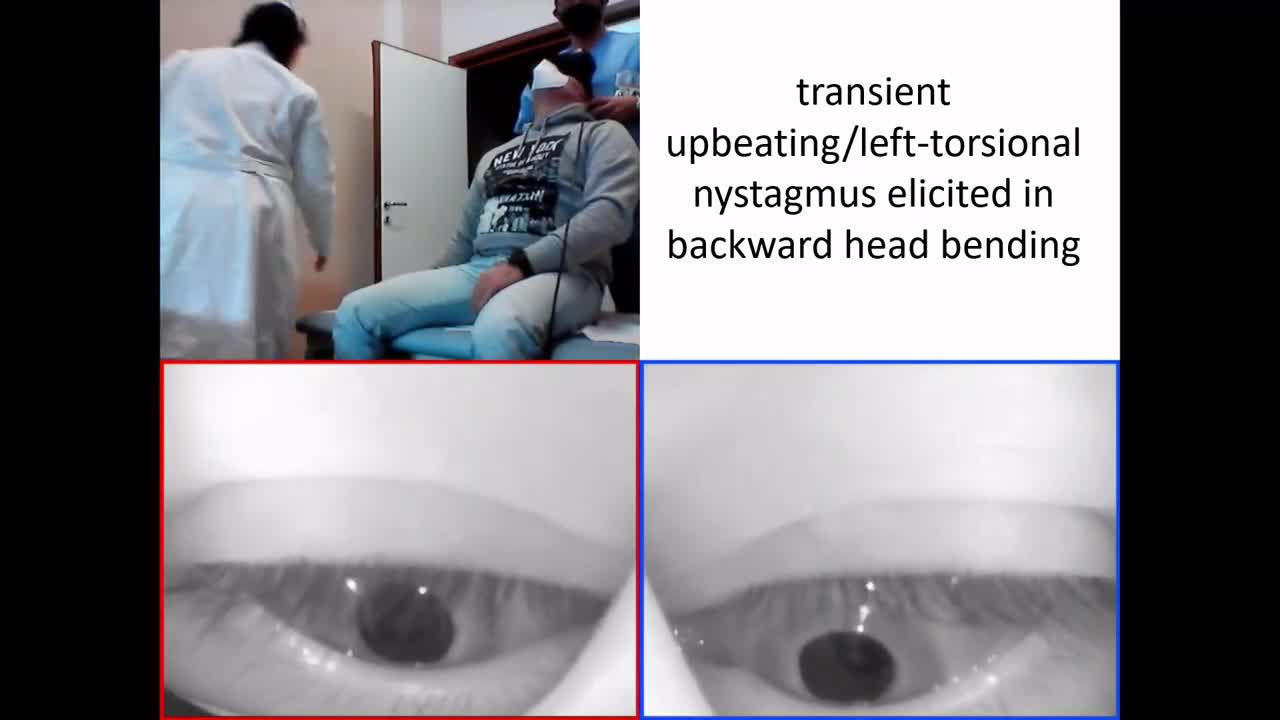
Video 2. A case of left LHS with PSC-BPPV following AUVP. No spontaneous nystagmus is detected on video-Frenzel goggles, while paretic right-beating nystagmus is elicited by skull vibrations (consistent with previous left AUVP). Bending the head backward along the pitch plane, transient upbeating/left-torsional nystagmus is detected (consistent with left PSC-BPPV). Upbeating/left-torsional paroxysmal positional nystagmus is elicited in left Dix Hallpike test positioning
- In cases of LSH with HSC-BPPV, the supine head roll test should lead to a horizontal paroxysmal direction-changing positional nystagmus in geotropic (in case otoconia settle the non-ampullary arm) or in apogeotropic form (when otoconia settle the ampullary arm). It has been also described a horizontal persistent apogeotropic direction-changing positional nystagmus in case of HSC cupulolithiasis (Video 3). In case of AUVP and simultaneous HSC-BPPV, positional nystagmus superimpose the baseline ongoing spontaneous nystagmus enhancing, mitigating or transiently reversing the spontaneous nystagmus.
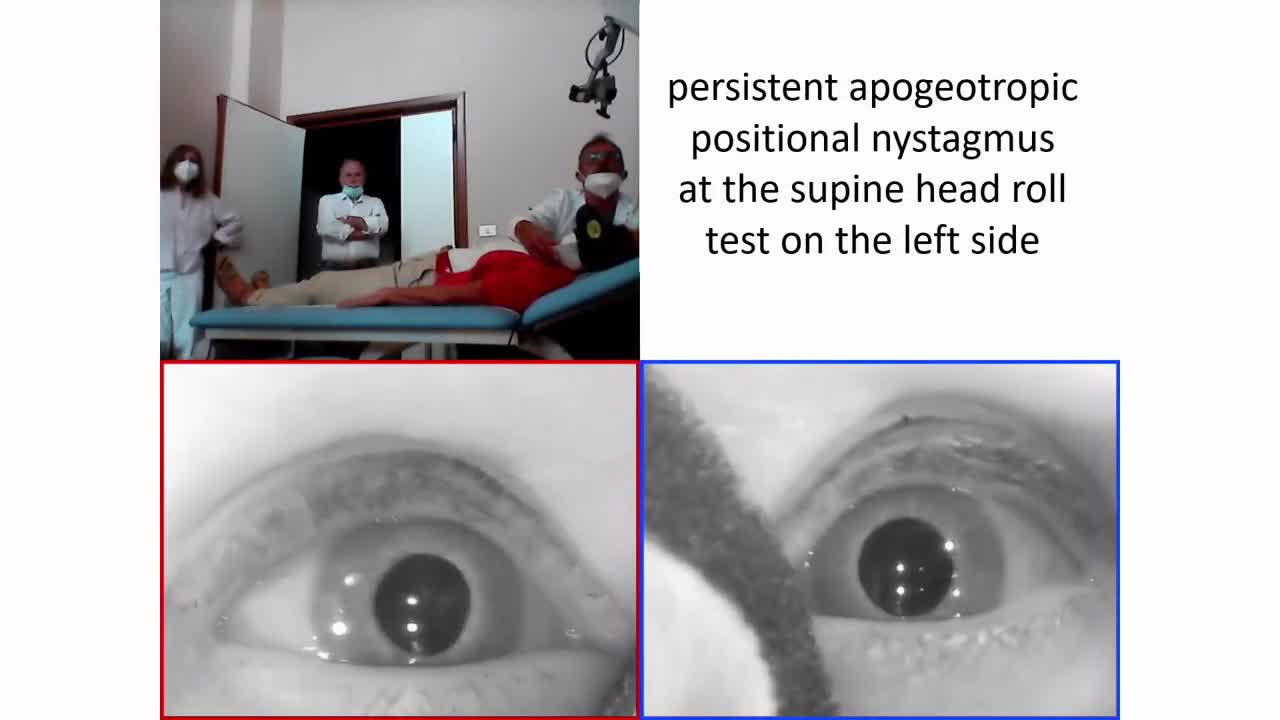
Video 3. A case of right LHS with HSC cupulolithiasis following AUVP. No spontaneous nystagmus is detected with video-Frenzel goggles, while paretic left-beating nystagmus is elicited by skull vibrations and head shakings (consistent with right AUVP). At the supine head roll test, persistent apogeotropic direction-changing positional nystagmus with stronger amplitude on the left side (consistent with right HSC-cupulolithiasis) is detected.
AudiologyOnline: Which is the typical instrumental picture?
Andrea Castellucci: The instrumental battery test measurements depend on the lesion extent and on the time elapsed from the onset of symptoms and the examination. Generally, the typical scenario is consistent with unilateral vestibular loss involving the superior branch of the VN, and include (Figure 3):
- Caloric testing showing unilateral canal paresis (CP)
- Video HIT (vHIT) exhibiting a lesion pattern usually involving the HSC and the ASC on the affected side, sparing the PSC
- Cervical VEMPs showing symmetrical potentials consistent with spared saccular function on the lesioned side
- Ocular VEMPs showing asymmetrical potentials consistent with absent / reduced utricular function on the lesioned side
- Pure-tone audiometry showing symmetrical hearing function.
It's crucial to distinguish LHS from other vestibular disorders to ensure appropriate management.
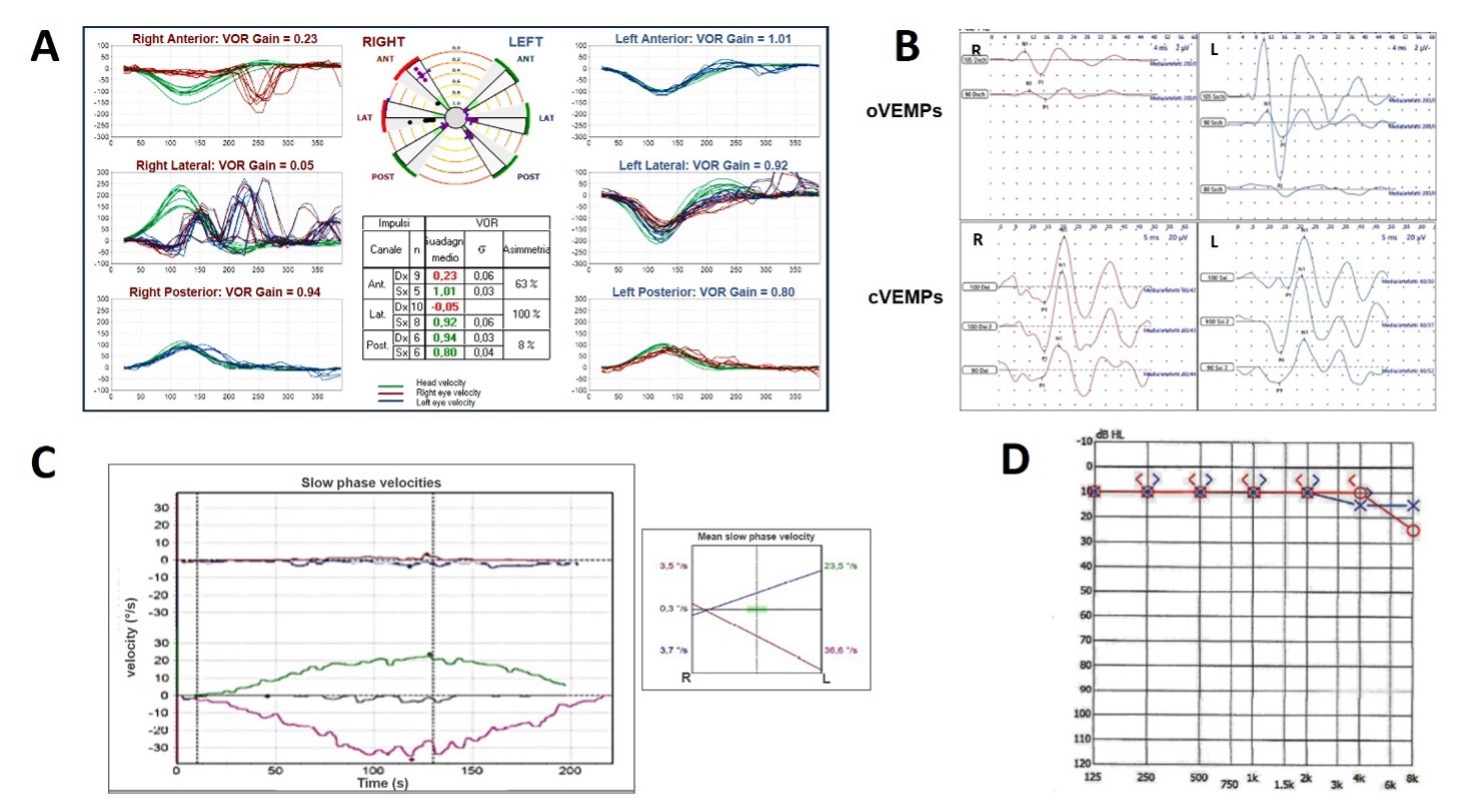
Figure 3. Typical instrumental assessment in case of LHS on the right side. (A) vHIT with reduced VOR gain values for the right HSC and LSC with spared function for the PSC. (B) ocular VEMPs showing reduced potentials on the right side and cervical VEMPs showing symmetrical amplitudes. (C) Caloric test with right hyporeflexia. (D) Pure tone audiometry showing normal hearing.
AudiologyOnline: Is the function of the SC involved by BPPV always spared in case of Lindsay-Hemenway Syndrome?
Andrea Castellucci: Even though positional nystagmus originating from the activation of canal afferents should theoretically imply a functionally active status of the affected SC, cases of LHS with BPPV involving hypoactive SC at the vHIT have been described in literature (both PSC-BPPV in case of complete vestibular lesion and HSC-BPPV following an ipsilesional AUVP). Several explanations have been proposed to clarify this paradox. Regardless of the underlying pathomechanism, the extent and severity of an acute peripheral lesions can vary, and different vestibular receptor subtypes may recover at different rates. Even within a single end-organ, the afferents or receptors may be either asymmetrically impaired or exhibit distinct recovery timelines and dynamics. In fact, SC receptors contain both type I and type II hair cells.
- Type I hair cells are located centrally in the ampullary crest and are connected to large afferents discharging irregularly as they respond to high-frequency stimuli and rapid head movements (“phasic afferents”).
- On the other hand, type II hair cells are found at the periphery and are linked to smaller afferents discharging regularly, as they are sensitive to low-frequency stimuli and slower head movements (“tonic afferents”).
Unfortunately, while both types of afferents in the HSC can be effectively assessed using standard clinical tests, as caloric test evaluates the vestibulo-ocular reflex (VOR) in the low frequency domain (0.03 Hz) and the vHIT measures high-frequency (5–7 Hz) responses, no reliable device can measure the activity of vertical SCs in the low frequency domain. Therefore, the following putative pathomechanisms accounting for a LHS involving a SC with functional loss at the vHIT still need to be proved
- It might be speculated that, in case of BPPV involving a SC that is functionally impaired according to vHIT data, the residual canal function following AUVP could be enough to generate paroxysmal nystagmus aligning with the plane of the involved SC.
- Conversely, it might be assumed that the vestibular insult in AUVP could selectively impair type I hair cells and/or phasic afferents sparing tonic sensors and afferents, thus allowing otoconia to elicit a positional nystagmus generated by low-acceleration endolymphatic flows.
- On the other hand, it might be hypothesized an asymmetrical recovery between the different subtypes of vestibular receptors / afferents, where vestibular sensors encoding low-frequency responses recover faster. Several studies have indirectly explored the differing recovery timelines of type I and type II canal afferents following AUVP by tracking vHIT and caloric results over time. These studies show that although both tests typically yield abnormal results at disease onset, their findings may diverge during the recovery phase. Notably, there is disagreement in the literature regarding which test demonstrates faster recovery. While some studies observed comparable improvements in vHIT asymmetry and CP within a relatively short follow-up period, other investigations found that HSC-VOR gain improve significantly at the vHIT over a longer follow-up while CP remained unchanged. In contrast, newer studies focusing on patients in the chronic stage of AUVP found that high-frequency canal responses remain impaired in a substantial portion of patients, while only a smaller part of them showed abnormal CP, highlighting a possible faster recovery of hair cells / afferents encoding low frequency stimuli.
AudiologyOnline: How to manage patients with Lindsay-Hemenway Syndrome?
Andrea Castellucci: Management involves addressing both phases:
- AUVP management through the use of pharmacological therapy (steroids and vestibular suppressant in the first days, followed by Betahistine and other products stimulation vestibular compensation) and vestibular rehabilitation therapy.
- BPPV management through CRMs depending on the involved canal. In case of PSC-BPPV, Epley or Semont’s CRM are usually the treatment of choice to relocate otoconia back to the utricle, while barbecue or Gufoni CRMs, followed by the Vannucchi's forced prolonged position on the healthy side, are effective for the treatment of geotropic HSC-BPPV. In case of apogeotropic HSC-BPPV, Appiani or Zuma CRMs are usually effective either to convert it into a geotropic variant or to directly resolve BPPV.
Studies have demonstrated high success rates with these maneuvers in LHS patients. Early recognition and appropriate management of LHS can significantly improve patient outcomes by effectively addressing both the acute vestibular insult and the subsequent BPPV.
References
- Allum JH, Cleworth T, Honegger F. Recovery of Vestibulo-Ocular Reflex Symmetry After an Acute Unilateral Peripheral Vestibular Deficit: Time Course and Correlation With Canal Paresis. Otol Neurotol. 2016;37:772-780. doi: 10.1097/MAO.0000000000001054.
- Armato E, Dumas G, Perottino F, Casteran M, Perrin P. Determination of Recovery by Total Restitution or Compensation Using Multifrequency Vestibular Tests and Subjective Functional Scales in a Human Model of Vestibular Neuritis. Audiol Res. 2024;14:958-982. doi: 10.3390/audiolres14060080.
- Balatsouras DG, Koukoutsis G, Ganelis P, et al. Benign paroxysmal positional vertigo secondary to vestibular neuritis. Eur Arch Otorhinolaryngol. 2014;271:919-24. doi: 10.1007/s00405-013-2484-2.
- Bhattacharyya N, Gubbels SP, Schwartz SR, Edlow JA, El-Kashlan H, Fife T, et al. Clinical Practice Guideline: Benign Paroxysmal Positional Vertigo (Update). Otolaryngol Head Neck Surg. 2017;156(3_suppl):S1-S47. doi: 10.1177/0194599816689667.
- Casani AP, Cerchiai N, Navari E. Paroxysmal positional vertigo despite complete vestibular impairment: the role of instrumental assessment. Acta Otorhinolaryngol Ital. 2018;38:563-568. doi: 10.14639/0392-100X-1549.
- Castellucci A, Botti C, Bettini M et al.. Case Report: Could Hennebert's Sign Be Evoked Despite Global Vestibular Impairment on Video Head Impulse Test? Considerations Upon Pathomechanisms Underlying Pressure-Induced Nystagmus due to Labyrinthine Fistula. Front Neurol. 2021;12:634782. doi: 10.3389/fneur.2021.634782.
- Castellucci A, Botti C, Delmonte S, Bettini M, Lusetti F, Brizzi P, et al. Vestibular assessment in sudden sensorineural hearing loss: Role in the prediction of hearing outcome and in the early detection of vascular and hydropic pathomechanisms. Front Neurol. 2023;14:1127008. doi: 10.3389/fneur.2023.1127008.
- Choi KD, Oh SY, Kim HJ, et al. Recovery of vestibular imbalances after vestibular neuritis. Laryngoscope. 2007;117:1307-1312. doi: 10.1097/MLG.0b013e31805c08ac.
- Comacchio F, Mion M, Armato E, Castellucci A. Sequential Vestibular Neuritis: Report of Four Cases and Literature Review. J Audiol Otol. 2021;25(2):89-97. doi: 10.7874/jao.2020.00360.
- Dumas G, Quatre R, Schmerber S. How to do and why perform the skull vibration-induced nystagmus test. Eur Ann Otorhinolaryngol Head Neck Dis. 2021;138(4):287-290. doi: 10.1016/j.anorl.2020.11.014.
- Eatock RA, Songer JE. Vestibular hair cells and afferents: two channels for head motion signals. Annu Rev Neurosci. 2011;34:501-34. doi: 10.1146/annurev-neuro-061010-113710.
- Epley JM. Human experience with canalith repositioning maneuvers. Ann N Y Acad Sci. 2001;942:179-91. doi: 10.1111/j.1749-6632.2001.tb03744.x
- Eza-Nuñez P, Fariñas-Alvarez C, Fernandez NP. Comparison of three diagnostic tests in detecting vestibular deficit in patients with peripheral vestibulopathy. J Laryngol Otol. 2016;130:145-50. doi: 10.1017/S0022215115003114.
- Faralli M, Ricci G, Manzari L, Zambonini G, Lapenna R, Pettorossi VE. Different time course of compensation of subjective visual vertical and ocular torsion after acute unilateral vestibular lesion. Eur Arch Otorhinolaryngol. 2021;278:2269-2276. doi: 10.1007/s00405-020-06312-0.
- Fu W, He F, Wei D, et al. Recovery Pattern of High-Frequency Acceleration Vestibulo-Ocular Reflex in Unilateral Vestibular Neuritis: A Preliminary Study. Front Neurol. 2019;10:85. doi: 10.3389/fneur.2019.00085.
- Goldberg JM. The vestibular end organs: morphological and physiological diversity of afferents. Curr Opin Neurobiol. 1991;1:229-35. doi: 10.1016/0959-4388(91)90083-j.
- Guo P, Zhao J, Jia G, Li H, Li W. Dynamic change of vestibular function and the long-term prognosis of vestibular neuritis. J Vestib Res. 2023;33:411-422. doi: 10.3233/VES-220104.
- Harada K, Oda M, Yamamoto M, Nomura T, Ohbayashi S, Kitsuda C. A clinical observation of benign paroxysmal positional vertigo (BPPV) after vestibular neuronitis (VN). Acta Otolaryngol Suppl. 1993;503:61-3. doi: 10.3109/00016489309128074.
- Hwang K, Kim BG, Lee JD, Lee ES, Lee TK, Sung KB. The extent of vestibular impairment is important in recovery of canal paresis of patients with vestibular neuritis. Auris Nasus Larynx. 2019;46:24-26. doi: 10.1016/j.anl.2018.05.009.
- Hemenway WG, Lindsay JR. Postural vertigo due to unilateral sudden partial loss of vestibular function. Ann Otol Rhinol Laryngol. 1956;65:692-706. doi: 10.1177/000348945606500311.
- Karlberg M, Hall K, Quickert N, Hinson J, Halmagyi GM. What inner ear diseases cause benign paroxysmal positional vertigo? Acta Otolaryngol. 2000;120:380-5. doi: 10.1080/000164800750000603.
- Kim HA, Hong JH, Lee H, et al. Otolith dysfunction in vestibular neuritis: recovery pattern and a predictor of symptom recovery. Neurology. 2008;70:449-53. doi: 10.1212/01.wnl.0000297554.21221.a0.
- Lee NH, Ban JH, Lee KC, Kim SM. Benign paroxysmal positional vertigo secondary to inner ear disease. Otolaryngol Head Neck Surg. 2010;143:413-7. doi: 10.1016/j.otohns.2010.06.905.
- Lee HJ, Kim SH, Jung J. Long-Term Changes in Video Head Impulse and Caloric Tests in Patients with Unilateral Vestibular Neuritis. Korean J Otorhinolaryngol-Head Neck Surg. 2019;62:23-27. doi:https://doi.org/10.3342/kjorl-hns.2017.01081.
- Libonati GA, Martellucci S, Castellucci A, Malara P. Minimum Stimulus Strategy: A step-by-step diagnostic approach to BPPV. J Neurol Sci. 2022;434:120158. doi: 10.1016/j.jns.2022.120158.
- Mandalà M, Santoro GP, Awrey J, Nuti D. Vestibular neuritis: recurrence and incidence of secondary benign paroxysmal positional vertigo. Acta Otolaryngol. 2010;130:565-7. doi: 10.3109/00016480903311278.
- Manzari L, Princi AA, De Angelis S, Tramontano M. Clinical value of the video head impulse test in patients with vestibular neuritis: a systematic review. Eur Arch Otorhinolaryngol. 2021;278:4155-4167. doi: 10.1007/s00405-021-06803-8.
- Manzari L, Graziano D, Zambonini G, Faralli M, Morone G, Tramontano M. The clinical course of vestibular neuritis from the point of view of the ocular vestibular evoked myogenic potential. J Laryngol Otol. 2022;136:129-136. doi: 10.1017/S0022215122000081.
- Martellucci S, Malara P, Pagliuca G, Castellucci A. Lindsay-Hemenway Syndrome Involving the Horizontal Semicircular Canal: Some Considerations Upon Residual Canal Afferents in BPPV Secondary to an Ipsilateral Acute Unilateral Vestibulopathy. Otol Neurotol. 2025. [Online ahead of print] doi: 10.1097/MAO.0000000000004512
- Navari E, Casani AP. Lesion Patterns and Possible Implications for Recovery in Acute Unilateral Vestibulopathy. Otol Neurotol. 2020;41:e250-e255. doi: 10.1097/MAO.0000000000002476.
- Pogson JM, Taylor RL, Young AS, McGarvie LA, Flanagan S, Halmagyi GM, Welgampola MS. Vertigo with sudden hearing loss: audio-vestibular characteristics. J Neurol. 2016;263(10):2086-96. doi: 10.1007/s00415-016-8214-0.
- Schmid-Priscoveanu A, Böhmer A, Obzina H, Straumann D. Caloric and search-coil head-impulse testing in patients after vestibular neuritis. J Assoc Res Otolaryngol. 2001;2:72-88. doi: 10.1007/s101620010060.
- Starkov D, Strupp M, Pleshkov M, Kingma H, van de Berg R. Diagnosing vestibular hypofunction: an update. J Neurol. 2021;268:377-385. doi: 10.1007/s00415-020-10139-4.
- Striteska M, Skoloudik L, Valis Met al. Estimated Vestibulogram (EVEST) for Effective Vestibular Assessment. Biomed Res Int. 2021;2021:8845943. doi: 10.1155/2021/8845943.
- Strupp M, Bisdorff A, Furman J, et al. Acute unilateral vestibulopathy/vestibular neuritis: Diagnostic criteria. J Vestib Res. 2022;32:389-406. doi: 10.3233/VES-220201.
- Türk B, Akpinar M, Kaya KS, Korkut AY, Turgut S. Benign Paroxysmal Positional Vertigo: Comparison of Idiopathic BPPV and BPPV Secondary to Vestibular Neuritis. Ear Nose Throat J. 2021;100:532-535. doi: 10.1177/0145561319871234.
- von Brevern M, Bertholon P, Brandt T, et al. Benign paroxysmal positional vertigo: Diagnostic criteria. J Vestib Res. 2015;25:105-17. doi: 10.3233/VES-150553.
- Waissbluth S, Becker J, Sepúlveda V, Iribarren J, García-Huidobro F. Benign Paroxysmal Positional Vertigo Secondary to Acute Unilateral Peripheral Vestibulopathy: Evaluation of Cardiovascular Risk Factors. J Int Adv Otol. 2023;19:28-32. doi: 10.5152/iao.2023.22703.
- Zellhuber S, Mahringer A, Rambold HA. Relation of video-head-impulse test and caloric irrigation: a study on the recovery in unilateral vestibular neuritis. Eur Arch Otorhinolaryngol. 2014;271:2375-2383. doi: 10.1007/s00405-013-2723-6.
Resources for More Information
For more information, visit https://www.inventis.it/en-na


