Maternal consumption of alcohol during pregnancy results in a range of developmental abnormalities known as fetal alcohol spectrum disorders. Fetal alcohol syndrome is the most severe manifestation within this continuum and can affect all parts of the auditory system along the auditory pathway. This review describes the effects of prenatal alcohol exposure on the peripheral and central auditory nervous systems and presents literature on the possible pathogenesis and mechanisms of damage.
Introduction
Characteristics of fetal alcohol syndrome
Maternal consumption of alcohol during pregnancy may lead to a number of birth defects, mental retardation, and neurodevelopmental disorders in the offspring, which are classified under the broad category of fetal alcohol spectrum disorders (FASD). In the early 1970s, a cluster of classic manifestations of prenatal alcohol exposure were recognized as a clinical entity called fetal alcohol syndrome (FAS). Based on 245 case reviews, Clarren and Smith (1978) first described the classic craniofacial features of children with FAS: microcephaly, short palpebral fissures, a hypoplastic philtrum, thin upper vermilion border, and, during infancy, retrognathism. Moreover, these investigators indicated that presentations of microcephaly and short palpebral fissures may predict reduced brain and eye size and that the latter physical feature may be the hallmark of FAS diagnosis. Of the remaining manifestations, the short and upturned nose and hypoplastic maxilla may be seen in as many as 50% of the affected cases and cleft lip and/or palate may occur less frequently (Clarren & Smith, 1978).
Other terminologies used to describe effects of fetal alcohol exposure
The term fetal alcohol effects (FAE) was used originally to describe the effects of prenatal alcohol exposure in animal studies; however, it was quickly accepted by clinicians to describe children with a number of problems such as growth deficiency, behavioral mannerisms, and delays in motor and speech performance, but who otherwise did not display the full complement of FAS characteristics (American Academy of Pediatrics, 2000). Thus, the term was used to describe any condition that was considered to be secondary to in utero alcohol exposure (Streissguth et al., 1994). This led to considerable variability in defining and interpreting data across clinics and comparing research studies. Therefore, in 1996, the Institute of Medicine proposed the use of the terms alcohol related neurodevelopmental disorders (ARND) and alcohol related birth defects (ARBD) to define maternal history of alcohol use (substantial regular intake or episodic heavy drinking) and validate clinical outcomes of exposure (Institute of Medicine, 1996). The proposed new terminology subsequently led to the development of a classification system that used both pathophysiology and history of prenatal alcohol exposure to classify FAS. The most popular classification system now in use is the 4-digit diagnostic code that was developed by the Washington State FAS diagnostic and prevention network in 1997 (FAS DPN; Astley & Clarren, 1997).
Medical diagnosis of FAS - the 4-digit diagnostic code
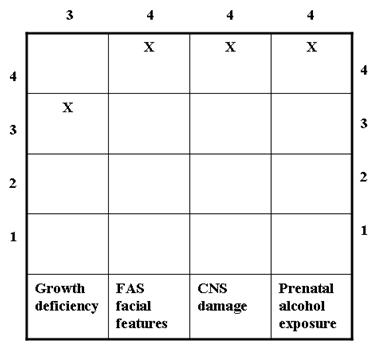
As the name suggests, the 4-digit diagnostic code incorporates a 4-digit scale to define the severity of the four classic FAS features: 1) growth deficiency, 2) FAS facial morphology, 3) central nervous system (CNS) damage/dysfunction, and 4) prenatal exposure to alcohol. Such a classification system accommodates differences in the magnitude of expression of the classic features, minimizes variability in clinical data, and enables comparisons across research studies (Astley & Clarren, 1997). Each feature is gauged independently on a 4-point Likert scale, where '1' reflects a completely normal finding, '4' represents the presence of a classic FAS feature, and scores of '2' and '3' represent intermediate manifestations between clearly typical and atypical (Astley & Clarren, 2000). The Likert scale is a psychometric scale (named after psychologist Rensis Likert in 1932) in which the step intervals correspond to level of measurement. However, these intervals do not necessarily measure degree of severity, but rather allude to the degree of confidence that a specific FAS feature is present. The 4-digit diagnostic code for a patient is typically derived after a thorough evaluation by a group of interdisciplinary professionals including a physician, psychologist, occupational therapist, speech-language pathologist, audiologist (less frequently involved), social worker, and family advocates (FAS DPN, 1997).
FAS refers to a patient who shows either '4s' in all 4 diagnostic categories or a combination of two '4s' and two '3s' on the diagnostic features (Astley & Clarren, 1997; 2000). The 4-digit diagnostic code for a typical FAS patient with a rating of 3, 4, 4, 4 on the 4 diagnostic categories would appear as follows (FAS DPN, 1997):
Prevalence of prenatal alcohol exposure and FAS
Data from the most recent behavioral risk factor surveillance programs analyzed by the Center for Disease Control and Prevention (CDC) indicates that approximately 1% of pregnant women (18-44 years) engaged in binge or frequent drinking during 2002 (binge drinking:> 5 drinks on one occasion; frequent drinking:> 7 drinks per week or binge drinking; CDC, 2004). Prevalence estimates for women who might become pregnant were even more dire, rising to 12.4% and 13.1% for binge and frequent drinking, respectively. In the latter group, prevalence of binge drinking was greatest at 19.4% in the 18-24 year olds, dropping to 13.1% and 8.6% in the 25-34 and 35-44 years age range, respectively. Furthermore, 25.2% of these women also reported cigarette smoking during the same time frame (CDC, 2004).
Estimates of FAS vary significantly due to a number of reasons, but primarily due to incorrect diagnosis and/or treatment for FAS related disabilities (Institute of Medicine, 1996). A recent study that reviewed an in-school screening and diagnosis method to ascertain active cases of FAS indicated that the prevalence of FAS is at least 2-7 per 1,000 children born in typical mixed racial and socioeconomic populations in the United States. Moreover, when all levels of FASD are included, the prevalence may be as high as 2-5% in the United States (May et al., 2009). These numbers clearly indicate the need to improve and increase the efforts for disseminating information regarding adverse effects of alcohol use, particularly during pregnancy. Survey outcomes also indicate the urgent need for early and better identification of alcohol induced problems, so that intervention may be implemented early to ensure better clinical outcomes.
FAS and the peripheral auditory system
Conductive vs. sensorineural hearing loss
In multiple studies, children with FAS have been reported to show much higher rates of intermittent hearing loss due to recurrent middle ear infections (75-93%) compared to the general pediatric population (12%). The FAS findings are more consistent with middle ear disorder prevalence rates in children with craniofacial anomalies such as cleft lip/palate (70%) and Down syndrome (62%; Church & Gerkin, 1988; Church et al., 1997; Rossig et al., 1994). However, the risk for middle ear disorders in children with cleft palate and Down syndrome is well recognized, and these populations receive early intervention services. In contrast, limited recognition and dissemination of information on the link between FAS and middle ear disorders has generally precluded children with FAS from receiving targeted early intervention services (Church & Gerkin, 1988).
A small percentage of FAS children have also been shown to display mild to moderate sensorineural hearing loss. Results from two studies conducted a decade apart by the Fetal Alcohol Research Center at Wayne State University Medical Center showed the prevalence rates of bilateral sensorineural hearing loss in FAS children to be 29% and 27%, respectively (Church & Gerkin, 1988; Church, et al., 1997). As seen with conductive hearing loss, prevalence rates for sensorineural hearing loss in FAS children are more consistent with those reported in children with craniofacial abnormalities like overt cleft palate (47%), submucous cleft palate (26%;), and Down syndrome (24%; Church & Kaltenbach, 1997), rather than the general pediatric population (2%; Church & Gerkin, 1988; Church, et al., 1997).
In contrast to the above findings, Rossig et al. (1994) reported a prevalence of only 7% (2 of the 36) of the FAS children they tested for sensorineural hearing loss. However, 27 of the 36 children they examined were classified in the mild FAS category using the 4 point Majewski scale, which includes assessments on growth and development, facial dysmorphism, skeletal abnormalities, and other malformations (Rossig et al., 1994). Thus, these findings may be related to categorical skewing of data and may be more consistent with findings associated with milder cases of FAS.
Otoacoustic emissions
Although there is evidence of sensorineural hearing loss in FAS children, there are no reports pertaining to the status of the cochlea or otoacoustic emissions (OAEs). In a very preliminary study being conducted in our laboratory, we are measuring conventional and ultrahigh frequency hearing and distortion product otoacoustic emissions (DPOAEs) in FAS children (Katbamna et al., unpublished). Data obtained from eight FAS subjects in the 8-17 year age range so far indicates that FAS children without middle ear problems show a trend towards reduced hearing thresholds (mean decline of ~ 5 dB) in the conventional (3-8 kHz) and ultrahigh frequency (9-20 kHz) regions (mean decline of ~3-14 dB) in both ears as compared to age and gender-matched typically developing (TD) children. Likewise, comparison of DPOAEs in the 2-8 kHz range show a trend towards reduced echo levels in the 4-8 kHz range (mean decline of 3-8 dB, with identical noise floors) in FAS children as compared to their TD counterparts. Thus, differences in hearing in the high frequency regions seen in FAS children may be attributed to a reduction of DPOAEs or to cochlear outer hair cell dysfunction. These findings are consistent with the loss of outer hair cells and/or malformed stereocilia reported in cochlear analyses of mammalian models of FAS (see below; Church et al., 1996).
FAS and central auditory nervous system deficits
Auditory brainstem responses
Unfortunately, very few studies have evaluated the influence of FAS on the auditory brainstem and central nervous system (CNS). In one of the earliest studies, Pettigrew and Hutchinson (1984) measured auditory brainstem responses (ABRs) in six infants with evidence of excessive prenatal alcohol exposure. Three of these infants were full term, whereas the remaining three were born at 32-34 weeks after conception. ABRs were normal in two of the three full term babies, but they were abnormal in the third full term baby, as well as all three preterm babies. The most notable ABR abnormalities were poor reproducibility and poor definition or absence of wave V as compared to a control group of normal infants. These ABR abnormalities persisted for several weeks after birth. Although not mentioned in the results, the figures also showed significantly delayed waves III and V and interpeak intervals I-III and I-V. Because ABR wave latencies would be expected to be delayed in preterm babies, especially towards the tail end of the window (due to cumulative effect across the time window), these prolongations may be attributed to prematurity; however, the additive effects of prenatal alcohol exposure cannot be ruled out in the outcomes of this study.
Later studies by Rossig et al. (1994) and Church et al. (1997) did show ABR abnormalities in a small percentage of children with FAS. Rossig et al. (1994) measured ABR latency-intensity functions in a group of FAS children aged 2 months to 17.4 years. Six of the 36 children (17%) tested showed ABR abnormalities; four showed low amplitude or absent waves IV and V, one showed prolonged wave III, and one showed prolongation of all waves, including interpeak intervals. Note that these six children were also categorized in the moderate or severe FAS categories. The findings in the majority of the mild FAS cases were unremarkable. The results of ABR tests measured by Church et al. (1997) were similar in that 2 of the 13 FAS children (15%) in the 3-27 year age range showed significant prolongations of interpeak interval I-III and/or I-V in one or both ears. These studies clearly indicate that the severity of ABR abnormalities may be related to the severity of FAS manifestations. Furthermore, the results suggest that ABRs may provide an objective index of auditory brainstem problems and concomitant auditory learning problems that these children often experience (see below).
Auditory processing deficits
There are only two reports on auditory processing problems in FAS children. Church et al. (1997) used the Word Recognition with Ipsilateral Noise (WRIN) test and the Competing Sentence Test (CST) to assess discrimination in figure-ground or competing conditions in 12 children (3-26 years old) with FAS. The WRIN test was conducted at 0 and 10 dB signal-to noise ratio (SNR), where the primary message (words) was presented at equal hearing levels or at 10 dB SL referenced to the competing message (noise). The CST was administered with phonemically balanced short sentences (6-7 words) presented simultaneously (dichotically) at presumably equal sensation levels. All 12 children (100%) showed abnormal test outcomes for the WRIN test at the 0 dB SNR, and 3 of the 12 children (25%) could not perform the test at all, even though all children (except two) scored at the lower range of normal performance at 10 dB SNR and showed excellent word recognition in quiet. The outcomes of the CST assessment were similar in that 10 of the 12 children (83%) could not perform the test at all when directed to the right or left ear; the remaining two children could perform the test, but only when directed to the right ear. It is not clear, however, if the overall test outcomes were confounded by existing hearing loss, because all children showed conductive or mixed hearing loss and comparable control subjects were not enrolled in the study. Overall, the findings have clear implications for speech, language, and auditory learning. Early identification and intervention of hearing loss prior to or concurrent with identification and intervention of auditory processing deficits is important to enable age appropriate acquisition of speech-language and auditory skills.
The second study used SCAN, a screening test for central auditory processing, the Pitch Pattern Sequence (PPS) test, and the Duration Pattern Sequence (DPS) test to explore auditory processing capabilities of eight children in the 6-11 years age range diagnosed with FAS by a medical geneticist (Martin et al., 2001). All children were screened for normal hearing, middle ear systems, and nonverbal intelligence. Children with FAS showed the greatest deficits in the PPS, DPS, and the competing word subtest of SCAN; they showed no significant differences in the filtered word and auditory figure-ground subtests of SCAN when compared to an age-matched control group of subjects. The authors concluded that children with FAS show 'scattered' auditory skills and a variety of auditory processing tasks need to be evaluated for appropriate identification and effective intervention.
Atypical functional lateralization
Although the CST outcomes described above allude to a functional bias towards the right ear in two children who could perform the test (Church et al., 1997), there is scant information pertaining to right or left ear dominance, especially in dichotic auditory tasks, in FAS children. Because children with FAS have repeatedly been shown to display abnormalities of the corpus callosum (the largest commissural fibers that connect the right and left hemispheres) on magnetic resonance imaging (MRI; e.g., Bookstein et al., 2002; 2007; Sowell et al., 2001), these children have long been suspected to be at risk for atypical hemispheric specializations, especially in the areas of speech, language, and hearing. A recent study that examined sensory-motor preferences and language lateralization in FAS children reported that these children displayed increased nonright-handedness, even though there were no differences in the preferential use of foot, eye, and ear, compared to a group of TD children (Domellof et al., 2009). Moreover, children with FAS showed greater number of right ear errors during a dichotic listening test, indicating that children with FAS may present with functional asymmetries that are not well established (e.g., reduced right ear advantage) or that are reversed (e.g., left ear advantage) compared to TD children. These investigators noted that children with altered functional lateralization due to developmental disabilities show similar functional asymmetries. Furthermore, prenatal brain injury due to alcohol exposure may alter functional organization due to a disruption in interhemispheric transfer (Domellof et al., 2009).
Speech-language, cognitive and other disabilities
Lack of or reversed hemispheric lateralization in the auditory mode is just one aspect of functional impairments that children with FAS experience. Depending on the amount, frequency, and timing of gestational alcohol abuse, developmental abnormalities and associated speech, language, hearing, and cognitive impairments vary substantially, but generally impose lifelong learning disabilities. Longitudinal follow-up of children with FAS into adulthood has shown the profound, pervasive, and permanent impact of the disorder in multiple domains (e.g., Streissguth et al., 1991; 1994); for example, multi-domain cognitive impairments including mathematical deficiencies, difficulty with abstract concepts (time and space, cause and effect, etc.), and problems with generalization from one circumstance to another identified early in life have been traced into adulthood. Likewise, behavioral problems including hyperactivity and impulsivity, memory deficits, maladaptive social functioning, and communication skills have been shown to pose significant problems throughout life (Streissguth et al., 1991; 1994). A study by Weinberg (1997) showed that children with FAS may present with deceptively good speech skills; however, compromised language skills often precludes adequate peer interaction, explaining at least in part the behavioral and social problems of individuals with FAS. Deficits in semantic and syntactic aspects of language use, along with poor oral-motor and articulation abilities, verbal learning, and short term memory have also been reported in early studies of American Indian FAS cohorts (Becker et al., 1990; Carney & Chermak, 1991).
The above findings indicate that clinically significant deficits in both receptive and expressive language abilities may undermine language development and comprehension in children with FAS (Church et al., 1997). Other factors such as mental impairment may further contribute to developmental delays, so that children with FAS may never be able to catch up with their TD peers over time (Streissguth et al., 1991; 1994). Mental impairments may also produce secondary disabilities like mental health problems, chemical dependency, inappropriate sexual behavior, and consequent legal problems in adults (Streissguth et al., 1991; 1994). However, recent evidence suggests that early diagnosis and intervention of FAS children, regardless of their intelligence quotients, may not only improve the chances of diminishing developmental delays, but also alleviate the occurrence of secondary disabilities (Streissguth et al., 1991; 1994).
FAS pathogenesis and mechanisms of damage
Cell death in the embryonic face and brain regions: A major contender for FAS pathogenesis
Although multiple aspects of the CNS have been shown to be altered by in utero exposure to alcohol in both humans and animal models, the typical facial features and CNS abnormalities associated with FAS have been attributed to excessive cell death in the embryonic face and brain regions (reviewed in Sulik, 2005). This loss of cell populations may not only explain the concomitant craniofacial anomalies, but also the accompanying middle ear problems observed in FAS cases.
Other vulnerable cell populations identified in animal models include those that are responsible for the formation of the ear and its nerve projections to the brain. In vertebrates, immediately after neural tube closure, the hindbrain or rhombencephalon is organized into seven segments called rhombomeres, and the ear emerges from a placode that forms at the junction of the third and fourth rhombomere (e.g., Lumsden, 1990; Guthrie, 1996). Studies that exposed mouse and chick embryos to alcohol have shown that the rhombomeres, neural crest cells, and placodes are highly sensitive to the effects of alcohol (Kotch & Sulik, 1992; Du & Hamre, 2001; Dunty et al., 2001; 2002; Sulik et al., 1988; Cartwright & Smith, 1995; Church et al., 1987). Neural crest cells are cells that delaminate from dorsal neural tube and overlying ectoderm regions and migrate into and contribute to the formation of the craniofacial structures, the cranial ganglia, the heart, and the thymus. Thus, widespread cell death within the hindbrain neuroepithelium, cranial neural crest cells, and placodes, leads to disruption of hindbrain segmentation and abnormalities of several cranial nerves including, V, VII-VIII, IX, and X. The abnormalities range from fusion or absence of the cranial ganglia to improper positioning or disorganization of nerve fibers (Dunty et al., 2001; 2002; Cartwright & Smith, 1995). Alcohol induced reduction in neuronal numbers within the auditory brainstem nuclei have also been documented (Pettigrew & Hutchinson, 1984; Mooney & Miller, 2001). Thus, teratogenic actions of alcohol on the embryonic precursor cells that contribute to the cochlea, auditory nerve, and brainstem may explain the auditory and audiologic manifestations observed in FAS.
Alcohol may also interfere with pattern formation and gene cascades important for
craniofacial development
There is growing evidence that prenatal exposure to alcohol disrupts pattern formation during early development in both humans and animal models. Pattern formation refers to the time- and location-specific order in which genes are turned on and off during development. When key regulatory genes do not turn on and off in a predictable manner, organization and positioning of specific cell types are disrupted, which leads to malformations of the craniofacial structures and other problems with normal growth. There is extensive literature on the effects of prenatal alcohol exposure on the eyes, forebrain, and midbrain, and the possible regulatory genes producing these effects. Examples include alcohol induced loss or downregulation (reduction) of sonic hedgehog (shh) in the forebrain, Fgf-8 in various organizing centers of the brain, and Pax6 in the forebrain and eyes; in addition, a number of genes regulating bone morphogenetic proteins involved in cell death cascades have been linked to craniofacial anomalies (reviewed in Sulik, 2005).
In the hindbrain, in contrast, there is no information on which signaling systems are targeted by alcohol exposure, in spite of abundant evidence of the precise and balanced interplay of multiple signaling systems. In our laboratory, we examined how alcohol exposure may change gene expression in the hindbrain of the frog, Xenopus laevis, especially the rostral hindbrain, which is critical for auditory function (Barsheff et al., 2010). In an earlier work, we showed that one of the connexin genes, connexin 43 (Cx43), turns on precisely when the auditory system and its neural connections are emerging from the hindbrain (Katbamna et al., 2004). Connexin genes facilitate cell-cell communication; therefore, mutation, loss, or downregulation of connexin genes could produce dysfunctional cells. This is demonstrated by the connexin 26 (Cx26) mutation which produces congenital cochlear hearing loss, because Cx26 is found in the cochlea. Cx43 is found in astrocytes (star shaped support or glial cells of the nervous system), and within the frog hindbrain Cx43 is strategically positioned within the centers of each rhombomere with processes reaching out into the outlying neuropil. Moreover, Cx43 glial cells alternate with other non-Cx43 expressing glial cells, which occupy the border regions of each rhombomere. This suggests that Cx43 glial cells may serve as guide post cells guiding neural development, whereas glial cells of rhombomeric boundaries may be involved in maintaining hindbrain segments (Katbamna et al., 2004).
In our alcohol exposure experiment, we predicted that if alcohol targeted the Cx43 gene, it would not only produce craniofacial anomalies in the frog, but it would also disrupt the spatiotemporal organization of Cx43 within the hindbrain (Barsheff et al., 2010). In fact, our results, showed alcohol induced neural and craniofacial anomalies in the frog that were associated with reduced facial and auditory-vestibular ganglia but not the ear and hindbrain structures. Furthermore, these changes occurred in concert with aberrant positioning of Cx43 cells, so that Cx43 clusters in the rostral hindbrain were displaced or absent, which in part explained the reduction of VII and VIII cranial ganglia and craniofacial anomalies. These findings clearly indicate that alcohol exposure interferes with timed gene expression, thereby targeting gene cascades that may be critical for the development of the hindbrain and its derivative ear and VII and VIII cranial nerves.
Summary
Children with FAS are at risk for developmental hearing loss of both conductive and sensorineural origin. Moreover, involvement of the central auditory nervous system may contribute to additional problems in the areas of speech-language and auditory learning. Thus, early identification and intervention are critical for successful clinical outcomes in not only speech-language and hearing areas, but also in the overall behavioral and social rehabilitation of FAS individuals.
References
American Academy of Pediatrics. (2000). Fetal alcohol syndrome and alcohol related neurodevelopmental disorders. Pediatrics, 106, 358-361.
Astley, S. J., & Clarren, S., K. (1997). The development of the fetal alcohol syndrome diagnostic and prevention network in Washington state. In: Streissguth, A., Kanter, J., eds. The challenge of fetal alcohol syndrome: Overcoming secondary disabilities. Seattle, Washington: University of Washington Press, pp. 40-51.
Astley, S. J., & Clarren, S., K. (2000). Diagnosing the full spectrum of fetal alcohol exposed individuals: introducing the 4-digit diagnostic code. Alcohol and Alcoholism, 35, 400-410.
Barsheff, T., Katbamna, B., & Ide, C.F. (2010). Fetal alcohol syndrome: Effects on the ear and cranial ganglia. American Academy of Audiology, San Diego, California.
Becker, M., Warr-Leeper, G.A., & Leeper, H.A. (1990). Fetal alcohol syndrome: A description of oral motor, articulatory, short term memory, grammatical and semantic abilities. Journal of Communication Disorders, 23, 97-124.
Bookstein, F.L., Sampson, P.D., Connor, P.D., & Streissguth, A.P. (2002). Midline corpus callosum is a neuroanatomical focus of fetal alcohol damage. Anatomical Record, 269, 162-174.
Bookstein, F.L., Connor, P.D., Huggins, J.E., Barr, H.M., Pimentel, K.D., & Streissguth, A.P. (2007). Alcoholism: Clinical and Experimental Research, 31, 868-879.
Carney, L.J., & Chermak, G.D. (1991). Performance of American Indian children with fetal alcohol syndrome on the test of language development. Journal of Communication Disorders, 24, 123-134.
Cartwright, M. M., & Smith, S. M. (1995). Increased cell death and reduced neural crest cell numbers in ethanol-exposed embryos: partial basis for the fetal alcohol syndrome phenotype. Alcoholism: Clinical and Experimental Research, 19(2), 378-386. 10
Centers for Disease Control and Prevention. (2004). Alcohol consumption among women who are pregnant or who might become pregnant - United States, 2002. Morbidity Mortality Weekly Report, 53, 1178-1181.
Church, M.W., Abel, E.L., Dintcheff, B.A., Gerkin, K.P., Gritzke, R., & Holloway, J.A. (1987). Brain-stem and cortical auditory evoked potentials in rats chronically exposed to alcohol in utero. Electroencephalography and Clinical Neurophysiology, Supplement, 40, 452-460.
Church, M.W., Abel, E.L, Kaltenbach, J.A., & Overbeck, G.W. (1996). Effects of prenatal alcohol exposure and aging on auditory function in the rat: preliminary results. Alcoholism: Clinical and Experimental Research, 20, 172-179.
Church, M.W., Eldis, F., Blakley, B., & Bawle, E. (1997). Hearing, language, speech, vestibular, and dentofacial disorders in fetal alcohol syndrome. Alcoholism: Clinical and Experimental Research, 21, 227-237.
Church, M.W. & Gerkin, K.P. (1988). Hearing disorders in children with fetal alcohol syndrome: Findings from case reports. Pediatrics, 82(2), 147-154.
Church, M., & Kaltenbach, J. (1997). Hearing, speech, language, and vestibular disorders in the fetal alcohol syndrome: A literature review. Alcoholism: Clinical and Experimental Research 21, 495-512.
Clarren, S. K., & Smith, D., W. (1978). The fetal alcohol syndrome. New England Journal of Medicine, 298, 1063-1067.
Domellof, E., Ronnqvist, L., Titran, M., Esseily, R., & Fagard, J. (2009). Atypical lateralization in children with fetal alcohol syndrome. Developmental Psychobiology, 51, 696-705.
Du, X., & Hamre, K. M. (2001). Increased cell death in the developing vestibulocochlear ganglion complex of the mouse after prenatal ethanol exposure. Teratology, 64, 301-310.
Dunty, Jr., W. C., Chen, S-Y., Zucker, R. M., Dehart, D. B., & Sulik, K. K. (2001). Selective vulnerability of embryonic cell populations to ethanol-induced apoptosis: implications for alcohol-related birth defects and neurodevelopmental disorder. Alcoholism: Clinical and Experimental Research, 25(10), 1523-1535.
Dunty, Jr., Zucker, R. M., & Sulik, K. K. (2002). Hindbrain and cranial nerve dysmorphogenesis result from acute maternal ethanol administration. Developmental Neuroscience, 24, 328-342.
Guthrie, S. (1996). Patterning the hindbrain. Current Opinions in Neurobiology, 6, 41-48. Institute of Medicine. (1996). Fetal alcohol syndrome: Diagnosis, epidemiology, prevention and treatment: Executive summary. Retrieved September 3, 2010, from www.comeover.to/FAS/IOMsummary.htm
Katbamna, B., Jelaso, A. M., & Ide, C. F. (2004). Connexin 43 expression in glial cells of developing rhombomeres of Xenopus laevis. International Journal of Developmental Neuroscience, 22, 47-55. 11
Kotch, L. E., & Sulik, K. K. (1992). Patterns of ethanol induced cell death in the developing nervous system of mice: Neural fold states through the time of anterior neural tube closure. International Journal of Developmental Neuroscience, 10, 273-279.
Likert. R. (1932). A technique for the measurement of attitudes. Archives of Psychology, 140, 1-55.
Lumsden, A. (1990). The cellular basis of segmentation in the developing hindbrain. Trends in Neuroscience 13, 329-335.
Martin, S.T., Baxter, B.M., McCormack, D.R., & Gerard, C.B. (2001, February 19). Performance of children with fetal alcohol syndrome on selected tasks of central auditory processing. AudiologyOnline. Web publication 212. Direct URL: www.audiologyonline.com/articles/article_detail.asp?article_id=212. Retrieved September 20, 2010 from eLearning on www.audiologyonline.com.
May, P.A., Gossage, J.P., Kalberg, W.O., Robinson, L.K., Buckley, D., Manning, M., & Hoyme, H.E. (2009). Prevalence and epidemiological characteristics of FASD from various research methods with an emphasis on recent in-school studies. Developmental Disabilities Research Reviews, 15, 176-192.
Mooney, S., M., & Miller, M. W. (2001). Episodic exposure to ethanol during development differentially affects brainstem nuclei in the macaque. Journal of Neurocytology, 30, 973-982.
Pettigrew, A.G., & Hutchinson, I. (1984). Effects of alcohol on functional development of the auditory pathway in the brainstem of infants and chick embryos. Ciba Foundation Symposium 105, 26-46.
Rossig, C., Wasser, St., & Oppermann, P. (1994). Audiologic manifestations in fetal alcohol syndrome assessed by brainstem auditory-evoked potentials. Neuropediatrics 25, 245-249.
Sowell, E.R., Thompson, P.M., Mattson, S.N., Tessner, K.D., Jernigan, T.L., Riley, E.P., & Toga, A.W. (2001). Voxel-based morphometric analysis of the brain in children and adolescents prenatally exposed to alcohol. Neuroreport, 12, 515-523.
Streissguth, A.P., Aase, J.M., Clarren, S.K., Randels, S.P., LaDue ,R.A., & Smith, D.F. (1991). Fetal alcohol syndrome in adolescents and adults. The Journal of American Medical Association, 265, 1961-1967.
Streissguth, A.P., Barr, H.M., Sampson, P.D., & Bookstein, F.L. (1994). Prenatal alcohol and offspring development: the first fourteen years. Drug and Alcohol Dependence, 36, 89-99.
Sulik, K. K. (2005). Genesis of alcohol-induced craniofacial development. Experimental Biology and Medicine, 230, 366-375.
Sulik, K. K., Cook, C. S., & Webster, W. S. (1988). Teratogens and craniofacial malformations: relationships to cell death. Development (supplement), 103, 213-231.12
Weinberg, N.Z. (1997). Cognitive and behavioral deficits associated with parental alcohol use.
Journal of the American Academy of Child and Adolescent Psychiatry, 36, 1177-1186.


