Introduction
Otitis media with effusion is relatively asymptomatic. Although it can be associated with as much as a mild to moderate conductive hearing impairment, it is unaccompanied by middle-ear inflammation, otalgia, and fever, which are the typical symptoms of acute otitis media. In children, the high prevalence of otitis media with effusion, often called the silent disease, underscores the need for pediatric acoustic-immittance screening. In the Netherlands, a longitudinal study assessing the prevalence of unilateral and bilateral otitis media with effusion was performed on 150 healthy infants and 100 high-risk infants from the neonatal intensive care unit (NICU) who were followed from birth through 24 months of age (Engel, Anteunis, Voloviks, Hendriks, & Marres, 1999). The results revealed that the prevalence of otitis media with effusion increased from 19% at birth to a peak of 49% at 9-12 months in healthy born babies and from 4% to 59% in the NICU babies. The prevalence then declined to 37% and 47% in the healthy born and NICU babies, respectively, at 24 months of age. The frequency of occurrence of bilateral otitis media with effusion exceeded that of unilateral otitis media with effusion by a ratio of 3:2. Research shows that the prevalence of otitis media with effusion peaks again at about five years of age (Paradise, 1980;Zielhuis, Rach, van den Bosch, & van den Broek, 1990). In a cohort study done on infants followed from birth to 24 months of age in Finland, the prevalence of persistent otitis media with effusion was determined to be 4.4% (Alho, Oja, Koivu, & Sorri, 1995).
Early studies on high-frequency tympanometry with the 660 Hz probe tone in neonates and infants were done by Margolis and Popelka (1975) and Shurin, Pelton, and Finkelstein (1977). The use of high-frequency tympanometry with the 1000 Hz probe tone in neonates and infants was introduced by Thornton, Kimm, Kennedy, and Cafarelli-Dees (1993) and Meyer, Jardine, and Deverson (1997);the 1000 Hz tone was further examined by Kei and his colleagues in 170 healthy neonates in 2003. Acoustic-reflex testing with a 660 Hz probe tone in neonates and infants was introduced by Margolis and Popelka (1975) in 2-4 month old infants, and Weatherby and Bennett (1980) examined the acoustic reflex at probe-tone frequencies ranging between 220 to 2000 Hz in a group of 44 neonates. Rhodes et al. (1999) examined the ipsilateral acoustic reflex (IAR) for the 1000 Hz probe tone with 2000 Hz and broad-band noise (BBN) activators in NICU neonates with an average chronological age of 31 days.
Overview of Acoustic Admittance
Currently, available commercial acoustic-immittance devices generally measure the aural acoustic admittance rather than the aural acoustic impedance. Aural acoustic admittance (Ya) is the reciprocal of impedance;therefore, acoustic-admittance devices are measuring the acceptance of energy by the middle ear. The unit of Ya is mmho. The components of Ya are mass susceptance (-BM), which represents the ease of sound flow into an object with mass;stiffness susceptance (+BS), which represents the ease of sound flow into an object with stiffness;and conductance (G), which represents the ease of sound flow into friction. The net susceptance (BT) represents the sum of the mass and stiffness susceptances. The stiffness susceptance is represented on the positive ordinate and the mass susceptance is represented on the negative ordinate. Low stiffness susceptance represents high stiffness, and conversely, high stiffness susceptance indicates low stiffness. A net susceptance that is stiffness susceptance indicates that the system has more stiffness than mass. And a net susceptance that is mass susceptance indicates that the system has more mass than stiffness.
The resultant admittance represents the vector sum of the susceptance and conductance vectors. Thus, based on the Pythagorean Theorem, the magnitude of the admittance vector is as follows:
FORMULA 1:
Y2 = (BT)2 + G2, and so Y = {(BT)2 + G2}2.
If the net susceptance is stiffness, then the vector representation is as follows:
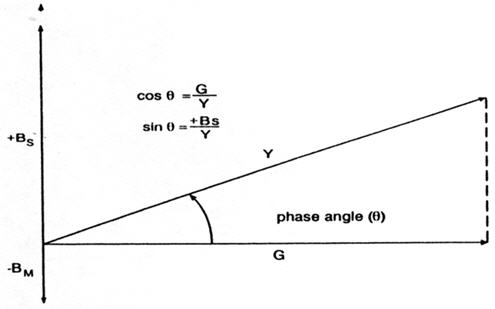
FIGURE 1. Relations among conductance (G), stiffness susceptance (+Bs), and mass susceptance (-Bm), with permission from Silman and Silverman (1984).
The phase angle can be computed based on cos θ = G/Y, sin θ = B/Y, or θ = arctan (B/G). A positive phase angle signifies that the net susceptance is stiffness susceptance, and a negative phase angle signifies that the net susceptance is mass susceptance.
Based on tympanometry, the compensated admittance can be determined by subtracting the admittance at a tympanometric tail (i.e., +200 or -400 daPa) from the peak admittance. Otherwise, Formula 1 (as shown above) can be used to determine the compensated admittance by manually computing the compensated B (peak B minus B at a tail pressure) and compensated G (peak G minus G at a tail pressure) to calculate Y.
The major advantage of measuring aural admittance rather than impedance during tympanometry is that with aural admittance, the sound pressure level is kept constant in the ear canal, which prevents the ear-canal volume from confounding the immittance measurement. Also, the amplitude of the admittance tympanogram can be expressed in absolute units (mmhos) whereas the amplitude of the impedance tympanogram can be expressed only in arbitrary, relative units.
Effects of Probe-Tone Frequency on Admittance
The effects of probe-tone frequency on admittance, its components, and the compensated phase angle in adults and healthy, full-term infants (average chronological age = 3 weeks) were investigated by Shahnaz, Miranda, and Polka (2008). In adults, when compensation was at the positive tail (+250 daPa), the compensated phase angle was positive at the low-mid probe-tone frequencies through 630 Hz, which is consistent with a stiffness-dominated middle ear. It was about 0° at 710 and 800 Hz, which is consistent with a middle ear at resonance (i.e., the mass and stiffness susceptances summing to yield a net susceptance of 0 mmho). Lastly, it was negative at frequencies over 800 Hz up to 1000 Hz, which is consistent with a mass-dominated middle ear. In contrast, in infants, the compensated phase angle was positive at 226 Hz (still showing a net susceptance that is stiffness, but much smaller than in adults), negative at the higher frequencies through 560 Hz (consistent with a mass-dominated ear), about 0° at 610 through 800 Hz (consistent with middle-ear resonance), and then negative again at the higher probe-tone frequencies through 1000 Hz (consistent with a mass-dominated ear). These findings of a positive compensated phase angle with a small stiffness susceptance (positive compensation) at 226 Hz in infants are inconsistent with other studies that show the infant ear is mass dominated at low frequencies (Holte, Cavanaugh, & Margolis, 1991;Meyer et al., 1997). Although many articles cite Holte et al. (1991) in support of the conclusion that the infant ear is mass-dominated at low frequencies;Holte et al. (1991) stated that "several" (but not the majority) of "ears of neonates...showed negative admittance phase angles at all frequencies indicating a mass-controlled system" (p. 20) and "this suggests a greater contribution of mass and/or resistive elements for the youngest infants than in older infants and adults" (p. 20).
Shahnaz et al. (2008) further showed that, in adults, susceptance (B) compensated at the positive tail was positive and increased with probe-tone frequencies in the low frequencies through 450 Hz (consistent with a net susceptance that is stiffness), then remained positive but decreased with probe-tone frequencies between 560 and 630 Hz (still consistent with a net susceptance that is stiffness), became 0 mmho at 710 Hz (resonant frequency), and then became increasingly negative with higher frequency tones (consistent with a net susceptance that is mass). In contrast, in the infant ear, the compensated susceptance was positive at 226 Hz (although much smaller in infants than in adults), slightly negative at 355 and 450 Hz (consistent with a net susceptance that is mass), essentially 0 mmho (middle ear at resonance) or slightly positive (small net susceptance that is stiffness) at 530 through 800 Hz, and then negative at higher frequencies through 1000 Hz (consistent with a net susceptance that is mass).
The compensated (at the positive tail) admittance and conductance in adults were positive, increasing with probe-tone frequency throughout the frequency range. Similar effects of probe-tone frequency on compensated admittance and conductance were observed in infants, although the values were smaller for infants than adults, particularly at the mid-to-high-frequency probe tones.
- Thus, in adults, the middle-ear changes from being stiffness dominated at the low probe-tone frequencies to being mass dominated at the high probe-tone frequencies. In infants, the net susceptance is a small positive value or near 0 mmho throughout the probe-tone frequency range, and the conductance is greater than the susceptance at all probe-tone frequencies, particularly at the higher probe-tone frequencies. The middle-ear resonant frequency is lower in infants than adults (Holte et al., 1991;Weatherby & Bennett, 1980).
Many investigators have documented the effects of maturation on the outer ear and middle ear. Such effects include the following: development of the osseous external auditory meatus during the first 12 months of life (Anson, Bast, & Richany, 1955;Anson & Donaldson, 1981);rapid increases in all dimensions of the external auditory meatus during the first 2 years of life, with further increases beyond 2 years up to 7 years of age (Keefe, Bulen, Arehart, & Burns, 1993;Saunders, Kaltenbach, & Relkin, 1983);increases in the size of the middle-ear cavity and mastoid with temporal bone growth and increases in the size of the antral and mastoid sinuses throughout childhood (Eby & Nadol, 1986;Anson & Donaldson, 1981;Saunders et al., 1983);fusion of the tympanic annulus with the temporal bone postnatally (Anson et al., 1955;Anson & Donaldson, 1981);increased orientation of the TM within the vertical plane, postnatally (Eby & Nadol, 1986);ossification of the bone marrow within parts of the malleus and incus that is followed by transformation into vascular channels by 25 months of age (Yokoyama, Ino, Kakizaki, & Murakami 1999);decrease in mass postnatally from resolution of residual mesenchyme in the middle ear with age (Yokoyama et al., 1992) or from disappearance of the amniotic fluid in the middle ear with age (Paparella, Shea, Meyerhoff, & Goycoolea, 1980);increase in middle-ear stiffness (according to Keefe et al., 1993, the middle-ear stiffness in infants less than 24 months of age represents 50% of that in adults);tightening of the ossicular joints (Anson & Donaldson, 1981);loosening of the coupling between the stapes and annular ligament as cited by Keefe et al. (1993);and decrease in external auditory meatal resistance during the first 24 months of age, especially during the first 12 months (Keefe, Fitzpatrick, Liu, Sanford, & Gorga, 2010).
Problems with Tympanometry in Infants Using Low-Frequency Probe Tones
Interpretation of the 226 Hz, low-frequency probe-tone tympanogram in infants is problematic because of the following:
- Flat 226 Hz tympanograms have been observed in some neonates and infants with normal middle ears (Baldwin, 2006;Rhodes, Margolis, Hirsch, & Napp, 1999). Rhodes et al. (1999) found that of their neonates who failed 226 Hz tympanometry, about 30% passed a transient otoacoustic emissions (TEOAE) screening (achieved a signal-to-noise ratio of at least 3 dB for at least 3 of the 4 frequencies tested), and approximately 50-60% passed a distortion product otoacoustic emissions (DPOAE) screening (depending on the DPOAE pass/fail criterion).
- The sensitivity of the 226 Hz tympanogram to the presence of middle-ear effusion is low. Normal or notched tympanograms have been observed with a low frequency tone in infants with confirmed middle-ear effusion (Marchant et al., 1986 for infants below 2 months of age;Paradise, Smith, & Bluestone, 1976 for infants below 7 months of age). In her group of 156 ears of 107 neonates (mean chronological age of 11 weeks) with temporary conductive hearing loss, Baldwin (2006) observed single-peaked or biphasic 226 Hz tympanograms with the tympanometric peak pressure (TPP) within ±150 daPa in 95% of ears. Baldwin's diagnosis of temporary conductive hearing loss was based on absent TEOAEs, a bone-conduction auditory brainstem response (ABR) threshold not greater than 30 dB HL, a wave V latency-intensity function for the air-conduction ABR consistent with conductive hearing impairment, and follow-up behavioral assessment consistent with the absence of permanent peripheral hearing impairment.
According to Paradise et al. (1976), the low sensitivity of low-frequency probe-tone tympanometry to identify otitis media with effusion can be explained by the distensibility of the walls of the external auditory meatus with air-pressure changes. In contradiction to the theory of Paradise et al. (1976), Margolis, Bass-Ringdahl, Hanks, Holte, and Zapala (2003) failed to observe monotonic increases in admittance with negative to positive air-pressure sweeps, which would have been evidence of decreased volumes at negative pressures and increased volumes at positive pressures. However, research shows that external auditory meatus distensibility does have an effect on the tails of the tympanogram (Holte, Cavanaugh, & Margolis, 1990;Meyer).
Whereas the middle-ear system of adults is stiffness dominated at a low frequency probe tone, the stiffness susceptance is much smaller in infants than adults;therefore, a low-frequency probe tone is not useful for evaluating the middle-ear system of infants. Furthermore, conductance is larger than susceptance in infants but not in adults (Shahnaz et al., 2008). Above 7 months of age, the sensitivity of low-frequency probe tone tympanometry to middle-ear effusion becomes high (Cantekin, Bluestone, Fria, Stool, Beery, & Sabo, 1980;Paradise, Smith, & Bluestone, 1976;Schwartz & Schwartz, 1980). - The 226 Hz tympanogram can be irregularly shaped or artifactual. Kei et al. (2003) reported that in a group of 122 healthy, full-term neonates who passed a transient otoacoustic emissions (TEOAE) screen, less than 49% of the tympanograms were single peaked at 226 Hz. In contrast, 92% were single peaked at 1000 Hz. Similar findings were obtained by Margolis et al. (2003). Sprague, Wiley, and Goldstein (1985) reported that 83% of the B and G tympanograms at 226 Hz were notched or double-peaked in a group of 44 healthy, full-term neonates. Shahnaz, Miranda, and Polka (2008) found that admittance tympanograms were single-peaked for 88% of their healthy, full-term babies (average chronological age of 3 weeks) at 1000 Hz but only for 42% at 226 Hz. Of their NICU babies who passed automated ABR testing at 35 dBnHL and TEOAE screening, 94% had single peaked admittance tympanograms at 1000 Hz versus only 13% at 226 Hz.
- Thus, in summary, the 226-Hz probe tone for tympanometry is problematic in infants because it can yield flat tympanograms in infants with normal middle ears, the sensitivity of such tympanograms to middle-ear effusion is low in infants, and the tympanometric shape can be irregular or artifactual in infants.
Acoustic-reflex testing using low-frequency probe tones is also problematic in infants. Studies on the acoustic reflex in neonates as a function of probe-tone frequency in neonates (Bennett, 1984;Bennett & Weatherby, 1982;Weatherby & Bennett, 1980) indicate the following:
- Acoustic reflexes for the 220 Hz probe tone and BBN activator are largely absent. The acoustic reflex is measured at the plane of the tympanic membrane and a mismatch exists between the low impedance (high compliance) of the tympanic membrane and the higher impedance of the middle ear;therefore, a change (due to the acoustic reflex) within the higher impedance of the middle ear is difficult to detect. Himelfarb, Popelka, and Shanon (1979) attribute the acoustic-reflex absence to high resistance preventing detection of a change in stiffness due to the acoustic reflex. Keith (1973) obtained similar findings in which they only obtained clear acoustic-reflex responses 30% of the time using a 100 dB HL 220 Hz probe tone at 500 Hz and 1000 Hz.
- The percent of acoustic reflexes that are present for infants increases with probe-tone frequency such that by 800 Hz, the frequency of present acoustic reflexes resembles that for adults. At probe-tone frequencies ≥ 800 Hz, 100% of the infants have present acoustic reflexes for the BBN activator. Using the 220 Hz probe tone with tonal activators, Jerger, Jerger, Mauldin, and Segal (1974) found absent contralateral acoustic reflexes in 93% of infants between 0 and 35 months at 90 dB HL and in 20% at 110 dB HL. For children between 60 and 71 months of age, the presence of absent acoustic reflexes declined to 67% at 90 dB HL and 5% at 110 dB HL.
- The threshold of the acoustic reflex declines as probe-tone frequency increases in infants, becoming equivalent to that in adults at 1200 Hz. Abahazi and Greenberg (1977) observed a decline in the mean acoustic-reflex threshold in infants from 1 month to 12 months of age for tonal and BBN activators with a 220 Hz probe tone, in which the mean BBN acoustic-reflex threshold was roughly 15 to 20 dB lower than the mean tonal acoustic-reflex thresholds. Robertson, Peterson, and Lamb (1968) observed that the mean contralateral acoustic-reflex thresholds for tonal activators (500, 1000, and 2000 Hz) with the 220 Hz probe tone declined with age from 18 to 36 months of age and remained elevated at 36 months of age in comparison with the mean acoustic-reflex thresholds for adults.
- In summary, acoustic reflexes for low-frequency probe tones are usually absent or elevated in infants. As probe-tone frequency increases, the frequency of occurrence of acoustic reflexes increases and the threshold of acoustic reflexes declines in infants. The high rate of absent reflexes or elevated acoustic reflexes when present in infants is related to the mismatch, in infant ears, between the low impedance of the tympanic membrane and the higher impedance of the middle ear.
Rhodes et al. (1999) found that of the 146 ears of NICU babies who passed the 1000 Hz tympanogram screen (pass was defined as a present peak on the susceptance or conductance tympanogram), approximately 8-20% failed another objective audiologic measure (ABR threshold, DPOAEs, or TEOAEs). Swanepoel et al. (2007) obtained admittance tympanograms with a 1000 Hz probe tone in 278 healthy neonates. They observed that 94% of the 16 ears with double-peaked tympanograms passed a DPOAE screen, which suggests that this tympanometric shape, as well as the single-peaked shape, may be consistent with normal middle-ear function in infants. Using DPOAEs (pass criterion was a signal-to-noise ratio ≥ 6 dB for at least 4 of 5 frequencies) as the gold standard, the false-positive rate for 1000 Hz tympanometry was 5% and the false-negative rate was 43% (a pass on 1000 Hz tympanometry was based on the presence of a discernable peak). Another 1000 Hz tympanometric shape that has been observed in healthy neonates is the flat-sloping that is, somewhat z-shaped, with admittance values essentially unchanged from +200 to 0 daPa and then increasingly negative from 0 daPa to negative daPa values (Kei et al., 2003). The TEOAE amplitudes are significantly lower for this tympanometric shape as compared with those for the single-peaked tympanogram. In Baldwin's (2006) group of babies with temporary conductive hearing loss, trough (negative peak) 1000 Hz tympanograms were obtained in 99% of infants;however, 11% of their 162 ears (104 babies) with normal middle-ear function also had this tympanometric shape.
The results of TEOAE screenings have been used as the gold standard to evaluate tympanometric findings, but TEOAE pass results have been observed for some pediatric and adult ears with middle-ear dysfunction (Driscoll, Kei, & McPherson, 2000;Kei et al., 2003;Margolis et al., 2003;Owens, McCoy, Lonsbury-Martin, & Martin, 1992). This suggests that TEOAEs are imperfect as a gold standard for middle ear function;however, ethical considerations generally preclude the use of an invasive myringotomy to confirm the presence or absence of middle-ear dysfunction to research the sensitivity and specificity of tympanometry (Baldwin, 2006;Kei et al., 2003;Shahnaz et al., 2008). Furthermore, TEOAEs are very sensitive to hearing loss in excess of 30 dB HL, particularly hearing loss of at least 40 dB HL;whereas DPOAEs generally are less sensitive to mild hearing loss and can sometimes be present even for hearing loss of 50-60 dB HL, especially with the use of higher level stimulation (Harris & Probst, 2002).
The criteria employed by Shahnaz et al. (2008) for a normal admittance tympanogram at 1000 Hz were based on compensated admittance (positive tail) and shape (single or double peaked, with a distance between the two maxima not exceeding 120 daPa). With double-peaked tympanograms, the peak admittance was calculated from the notch between the maxima. In contrast, Margolis et al. (2003) calculated the peak admittance from the highest peak in double-peaked tympanograms. In the study by Shahnaz et al. (2008), of the infants on the high-priority hearing registry (average chronological age of 6 weeks) with normal admittance tympanometric findings at 1000 Hz, 81.6% passed the TEOAE screen, 16.6% failed the TEOAE screen, and 1.8% could not be tested on the TEOAE screen. Thus, a pass on tympanometry does not rule out middle-ear dysfunction. Of the group of infants on the high-priority hearing registry with abnormal tympanometric results at 1000 Hz, 74.1% failed the TEOAE screen and 25.9% passed. Although tympanometric shape and compensated admittance have been examined by several investigators for the 1000 Hz admittance tympanogram in infants (Alaerts , Luts, & Wouters, , 2007;Kei et al., 2003;Margolis et al., 2003;Mazlan et al., 2007;Palmu, Puhakka, Huhtala, Takala, & Kilpi, 2001), the daPa distance between the maxima of a double-peaked tympanogram has not been evaluated by other investigators.
The TPP appears to be a less useful criterion for tympanometric assessment of infants. For example, for the 1000 Hz probe tone, Mazlan et al. (2007) obtained a very large standard deviation of 45 daPa for a mean TPP of 12.5 daPa at birth, and a standard deviation of 68 daPa for a mean TPP of -2 daPa at 6-7 weeks chronological age in a group of full-term, healthy neonates who passed automated ABR and TEOAE screening. These findings indicate extremely large intersubject variability in the TPP of healthy neonates and infants. Large standard deviations for the mean TPP for the 1000 Hz probe tone also were obtained by Swanepoel et al. (2007) for their groups of healthy neonates less than 1 week of age and 1-4 weeks of age and by Palmu et al (2001) for their groups of 1616 healthy 7-month-old infants and 1,223 healthy 24-month-old children who had normal pneumatic otoscopic findings.
For the compensated acoustic admittance at 1000 Hz in infants, Margolis et al. (2003) compensated at the negative tail because the mean compensated admittance was larger at the negative tail (-400 daPa) than at the positive tail (+200 daPa), facilitating better separation between normal and abnormal tympanograms. Shahnaz et al. (2008), however, noted that 0 mmho values at negative air pressures on the susceptance tympanogram for the 1000 Hz probe tone in neonates indicate ear-canal collapse and are not uncommon. Also, Kei, Mazlan, Hickson, Gavranich, and Linning (2007) noted greater difficulty in maintaining hermetic seals at -400 daPa, which led to an inability to obtain admittance compensated at the negative tail in some ears. In their study, test-retest reliability for admittance (calculated from the compensated susceptance and conductance components) was greater with positive than negative tail compensation. This was shown with an intra-correlation coefficient of 0.92 for positive tail compensation versus 0.58 for negative tail compensation. The directly measured admittance (not derived from the compensated susceptance and compensated conductance components) that was compensated at +200 daPa had a high intra-correlation coefficient of 0.89. The standard error also was much larger for the component-compensated admittance based on the negative tail (0.33) than for the component compensated admittance based on the positive tail (0.13) or the directly measured admittance (not derived from the susceptance and conductance components) compensated at the positive tail (0.12). These findings support compensation of admittance at the positive rather than negative tail in infants.
Kei et al. (2007) examined healthy, full-term neonates who were not at risk for hearing loss (Joint Commission on Infant Hearing, 2000) and who passed a TEOAE screen. They assessed the compensated, manually computed admittance for the 1000 Hz probe tone based on the compensated susceptance and conductance components as well as the traditional, directly measured compensated admittance that is not based on the compensated susceptance and conductance components. Obtaining the compensated Y based on the compensated B and G components avoids errors associated with possible differences in the phase angle between the peak admittance and tail admittance leading to potential errors associated with subtraction of the tail Y from the peak Y. In adults, for the low-frequency probe tone, both phase angles are very close to 90° because the susceptance is much greater than the conductance;therefore phase angle differences are small and subtraction of the admittance vector quantity at a tail value from the admittance vector quantity at TPP will not lead to significant error (Shanks, 1984). However, as Margolis & Hunter (2000) noted, the relation between B and G at 1000 Hz in infants differs from that seen in adults so subtraction of the tail admittance from the peak admittance could lead to errors. Kei et al. (2007) observed that the phase angles for a representative infant for the 1000 Hz probe tone not only varied with ear-canal air pressure (over a 28° range in the representative infant), but they were also substantially less than 90°, reflective of admittance with significant conductance as well as stiffness susceptance components. For the 1000 Hz probe tone in their group of healthy neonates, the mean compensated admittance based on the compensated susceptance and conductance components was significantly larger than the mean traditional admittance (not manually computed from the compensated B and G) compensated at either tympanometric tail. Kei et al. (2007) concluded that separation between normal and abnormal tympanograms would be better for compensated admittance derived from the compensated components than for the traditionally measured compensated admittance. They suggested future research to evaluate sensitivity and specificity for both approaches to calculate compensated admittance in infants.
Although tympanometric width has been investigated in infants and neonates (Alaerts et al., 2007;Kei et al., 2003;Palmu et al., 2001;Shahnaz et al., 2008), the measure has not commonly been employed in protocols for 1000 Hz tympanometry in this population. Kei et al. (2003) noted the difficulty in determining tympanometric width when the compensated admittance is small, and the normative 5th percentile for compensated admittance has been reported to be as low as 0.1 (Margolis et al., 2003). Additionally, Shahnaz et al. (2008) noted that many infant ears that failed a TEOAE screening had 1000 Hz admittance tympanograms with a notch between two maxima that was below both tails, suggestive of middle-ear effusion. Tympanometric width would be difficult to measure with this tympanometric shape, and also would be difficult to measure in the flat-sloping tympanogram described by Kei et al. (2003).
According to Kei et al. (2007), infant ear-canal walls cannot be modeled as solely susceptance, because 1) the distensibility of neonate ear-canal walls with ear-canal air-pressure changes, and 2) ear-canal walls have an osseous component that develops over the course of the first year of life. Thus, measures of admittance at extreme ear-canal air pressures may not be good estimates of the equivalent ear-canal volume (EECV) in neonates and infants, particularly at the negative tail where ear-canal collapse is a common observation. In their representative infant tested at 3 days after birth, the phase angles at +200 and -400 daPa were 66.5 and 40 degrees, respectively, showing that the admittance at 1000 Hz is far from being solely susceptance at these ear-canal air pressures.
In their group of 173 ears of 87 NICU babies (average chronological age of 31 days), Rhodes et al. (1999) found that of the ears with absent IARs for the 1000 Hz probe tone for the BBN (maximum intensity used was 80 dB SPL) and 2000 Hz (maximum intensity used was 90 dB SPL) tonal activators, approximately 52% passed the ABR screen. This suggests a high false-positive rate for ipsilateral acoustic-reflex testing at 1000 Hz in neonates. Swanepoel et al. (2007) observed the presence vs. absence of the IAR for the 1000 Hz probe tone and the 1000 Hz activator (maximum intensity of 100 dB HL) in a group of 278 ears from healthy, full-term neonates (1-28 days old). They obtained, based on DPOAE results, a high false-positive rate for the IAR and a sensitivity of 57%. Mazlan et al. (2009) observed present IARs for the 2000 Hz tonal and BBN activator in 91% of the 219 ears from healthy, full-term neonates (mean chronological age of 54 hours) with single-peaked 1000 Hz tympanograms and pass results on the TEOAE screen, suggesting high specificity for the acoustic reflex. Using DPOAE results as their gold standard, Swanepoel et al. (2007) found for their group of 278 healthy neonates a false-positive rate for IARs (in which a fail was defined as an absent acoustic reflex at 110 dB HL for the 1000 Hz tonal activator and 1000 Hz probe tone) of 10%;the false-negative rate was the same as that observed for tympanometry (43%).
- In summary, in relation to admittance testing with the 1000-Hz probe-tone in infants, the results of studies suggest the following:
- Double- and single-peaked tympanometric shapes generally are associated with normal middle-ear function
- Flat-sloping, z-shaped tympanograms are less likely than double- or single-peaked tympanograms to be associated with normal middle-ear function
- Trough-shaped tympanograms are more likely to be associated with middle-ear dysfunction than with normal middle-ear function
- TEOAEs represent an imperfect gold standard for assessment of test performance of acoustic-immmittance measures
- With double-peaked tympanograms, measurement of peak admittance has been calculated either from the highest peak or from the notch between the maxima
- In general, acoustic-admittance results within normal limits do not rule out middle-ear dysfunction
- The tympanometric peak pressure represents a less useful admittance measure than the other acoustic-admittance measures
- For calculation of the peak-compensated acoustic admittance, compensation at the positive tail has advantages to that at the negative tail: The former, as compared with the latter, avoids the adverse effects of ear-canal collapse at negative air pressures, has greater test-retest reliability, and smaller standard errors.
- Obtaining compensated admittance based on the vector sum of the compensated individual susceptance and conductance components avoids mathematical errors that can occur with subtraction of the admittance at a tail air pressure from the peak admittance as the phase angles associated with the tail and peak pressures may differ. Nonetheless, research is needed to examine test performance for the compensated admittance based on computation of the vector sum of the compensated susceptance and conductance values vs. compensated admittance measured directly without computation of the vector sum of the compensated susceptance and conductance values.
- Tympanometric width is a less useful admittance measure because of the difficulty in deriving tympanometric width from small compensated admittance values.
- Equivalent ear-canal volume is a less useful admittance measure because the measures of admittance are poor estimators of the EECV as infant ear-canal walls cannot be modeled solely as susceptance.
- The specificity of the ipsilateral acoustic reflex for the presence vs. absence of middle-ear dysfunction appears to be high when interpreted in conjunction with a single-peaked admittance tympanogram. In isolation, the ipsilateral acoustic reflex is associated with high false-positive and false-negative rates.
Proposed Acoustic Immittance Protocols in Infants and Children
In summary, numerous approaches have been employed for immittance assessment of infants (including newborns) and children;however, a paucity of research exists on the sensitivity and specificity of these measures (using various criterion levels) and protocols for the various age subgroups within the pediatric population. A multitude of recommendations for specific age groups across a wide variety of tympanometric parameters have been presented, but protocol recommendations for the age subgroups from newborns (healthy born and NICU) to adulthood are lacking. In light of the enormous developmental changes in the ear canal and middle ear throughout much of childhood—which are reflected in the marked changes in normative data with age and the large intersubject variability—the age groups on which recommendations are based should be narrowly defined. Furthermore, normative data as well as data on protocol performance within each specific age group are needed. Research is also needed to compare directly measured compensated admittance versus admittance based on the compensated conductance and suceptance components in ears with and without middle-ear effusion. The following proposed immittance protocols represent one interim perspective based on the results of the aforementioned studies on multifrequency tympanometry.
For the neonate and 1-7 months age groups, the proposed immittance protocol is based on the tympanometric shape for the 1000 Hz admittance tympanogram using compensated admittance (directly calculated rather than determined from the compensated susceptance and conductance components) based on the positive tail (for single- or double-peaked tympanograms). IAR testing is based on the 1000 Hz probe tone for tympanometric shapes that are flat sloping or trough/negative peaks. Admittance should be compensated at the positive rather than negative tail based on the findings of Kei et al. (2003) showing greater reliability and a smaller standard error for compensation at the positive tail. The recommendation of IAR testing for flat sloping or trough/negative peaks is based on the findings of Alaerts et al. (2007) showing that some infants who pass ABR and TEOAE screenings have flat sloping tympanograms. In addition, there is an absence of sensitivity and specificity data for trough/negative peaks. Silman and Silverman (1984) initially had proposed doing IAR testing with the 1000 Hz probe tone and BBN activator in infants. For IAR testing on any age group, as noted by some investigators (Sells, Hurley, Morehouse, & Douglas, 1997;Silman & Silverman, 1984;Silman, Silverman, & Arick, 1992), an acoustic-immittance device with a multiplexing circuit should be employed because it controls for additive and subtractive artifacts (Kunov, 1977;Lutman, 1980;Lutman & Leis, 1980). A multiplexing circuit involves alternation of the activating and probe-tone signals so that interaction between these signals does not occur. This is accomplished using a pulsed activator and a continuously-on probe tone, which effectively works like a pulsed probe signal because the probe microphone is alternated between being on and off. Grason Stadler, for example, has immittance devices with a multiplexing circuit. At this time, Interacoustics, for example, does not.
The immittance protocols proposed for the 7-24 months and 2-3 years age groups are similar to those described for the neonate and 1-7 months age groups, with the primary difference being the use of the 226 Hz rather than the 1000 Hz probe tone for tympanometric testing. Also, for these age groups, the admittance at the positive tail should be used to estimate the EECV, as their ears probably can be modeled as pure, or nearly pure susceptance at the positive tail, similar to the findings in adults. An elevated EECV beyond the 95th percentile is known to be possibly consistent with a tympanic-membrane perforation or a patent tympanostomy tube in a patient with a known tympanostomy tube. Additionally, IAR testing using both the 226 Hz and 1000 Hz probe tones and the 2000 Hz and BBN activators is proposed.
For the 3-11 years age group, the TPP is added to the protocol and IAR testing is done only for the 226 Hz probe tone with the 1000 Hz tonal activator.
Referral is based on the findings of acoustic-immittance testing and TEOAEs, as OAE testing already is part of the newborn hearing screening. Consistent with the findings that normal tympanometric results do not rule out middle-ear dysfunction, pass results for acoustic-immittance testing with failure on TEOAE screening may reflect conductive and/or sensorineural hearing impairment. Failure on immittance testing with pass findings on the TEOAE screening is suggestive of a slight/mild conductive hearing impairment.
In addition, the results of an ABR screening may enhance interpretation of the results of TEOAEs and immittance testing.
Neonates
Figure 2 shows the proposed protocol for immittance assessment of neonates. As the normative compensated admittance values are based on 5th percentiles (0.1 mmho for healthy born neonates and 0.2 mmho for NICU neonates) established by Margolis et al. (2003), the tympanometric parameters include a positive to negative air-pressure sweep and a pump speed of 600/200 daPa/s. These 5th percentiles are based on compensation at the positive tail (+200 daPa). Kei et al. (2007) also employed these parameters. According to Margolis et al. (2003), the peak admittance should be based on the higher peak in double-peaked tympanograms. Tympanometric shape should be classified as: flat-sloping, single- or double-peaked, flat (Kei et al., 2003), or trough/negative peak (Baldwin, 2006).
For IAR testing with the 1000 Hz probe tone, the BBN activator is proposed, with pass/fail based on Mazlan et al.'s (2009) 95th percentile for healthy babies and Rhodes et al.'s (1999) findings for NICU babies. The 95th percentile is 80 dB HL;therefore, the intensity range for IAR testing could be 50 - 80 dB HL, using 5-dB steps.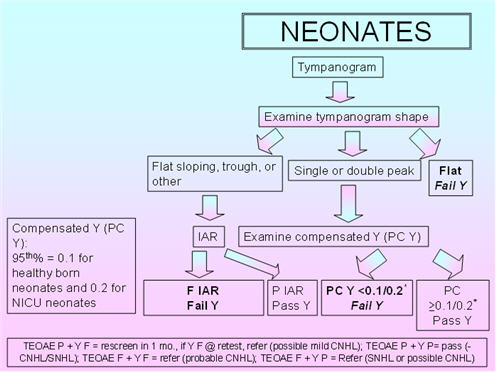
FIGURE 2. Proposed protocol for neonates.
1 Month - 7 Months
Figure 3 shows the proposed protocol for 1-7 month old infants. The tympanometric parameters are slightly modified in that the recommended pump speed is 400 daPa/s to 660/200 daPa/s. The proposed pump speed is based on Mazlan et al.'s (2009) use of 400 daPa/s, Alaerts et al.'s (2007) use of 500 daPa/s, and the fact that the commonly employed pump speed of 600/200 daPa/s is close to the former and latter speeds. The 5th percentile for compensated admittance (positive tail compensation) is based on Mazlan et al. (2009) for the younger age group and on Alaerts et al. (2007) for the older age group. The parameters for IAR testing are the 1000 Hz probe tone with the BBN and 2000 Hz activators. Studies show substantial declines in the acoustic-reflex threshold over this period for the 226 Hz probe tone;therefore, this probe tone does not yet represent an appropriate probe tone for this age group. Because normative IAR data for the 1000 Hz probe tone are sparse in this age group, it is proposed that a present IAR for the BBN or 2000 Hz activator should be considered as a pass and the absence of the IAR for both activators should be considered a fail.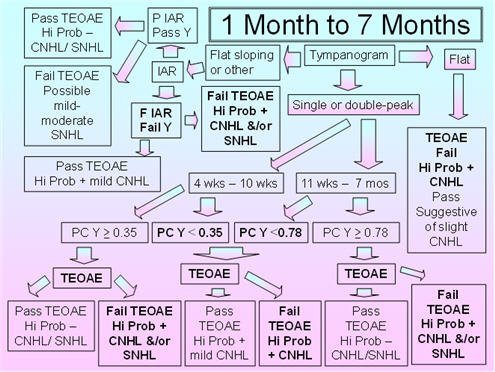
FIGURE 3. Proposed protocol for Infants between 1 month and 7 months of age.
7 Months - 24 Months
Figure 4 shows the recommended protocol for the 7 months to 24 months age group. Flat sloping admittance tympanograms for the 226 Hz probe tone have been observed in infants and young children between 3 months and 32 months of age (Alaerts et al., 2007);therefore, IAR testing is included in this protocol to resolve the pass/fail decision for the flat sloping tympanometric shape. Alerts et al. (2007) reported that the incidence of double-peaked tympanograms was 0% at 9-32 months;so, this tympanogram shape is not part of this proposed protocol. The 5th percentiles for compensated admittance are based on the findings of Palmu et al. (2001), whose 5th percentiles were the same for the 7 months and 24 months age groups (based on compensation at the positive tail);therefore, the recommended tympanometric parameters follow their procedures: positive to negative sweep and air pressure pump speed of 600/200 daPa/s. Because this age group shows large changes in acoustic reflex thresholds over time, and the data on the percentages of present IARs and on the IAR thresholds with the 226 Hz and 1000 Hz probe tones in this age group are sparse, it is proposed that IARs be obtained for both probe tones and the BBN and 2000 Hz activators. The presence of an IAR at 100 dB HL for either probe tone with either activator should be considered as a pass result, whereas the absence of the IAR for both probe tones and activator should be considered as a fail result.
The 95th percentiles for the EECV are based on the findings of deChicchis, Todd, and Nozza (2000) for the 6 months - 12 months age group and for the 1-2 years age group. The tympanometric parameters employed by deChicchis et al. (2000) are the same as those employed by Palmu et al. (2001).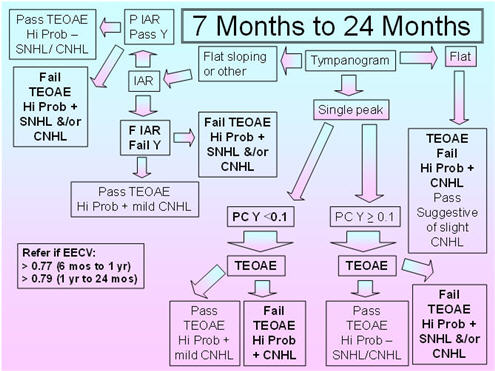
FIGURE 4. Proposed protocol for infants between 7 months and 24 months of age.
2 - 3 Years
Figure 5 shows the recommended protocol for the 2-3 years age group. As previously mentioned in the discussion of the 7-24 months protocol, flat sloping admittance tympanograms for the 226 Hz probe tone have been observed in infants and young children between 3 months and 32 months of age (Alaerts et al., 2007);therefore, IAR testing is included to resolve the pass/fail decision for this tympanometric shape. The 5th percentiles for compensated admittance are based on the findings of Roush et al. (1995), who obtained normative acoustic-immittance data for 88 African American children between 18-30 months of age with presumed normal middle-ear function based on pneumatic otoscopy. Normative data for the 2-3 years old group were obtained by deChicchis et al. (2000), but their 99 children were dispersed across five age groups, and the sample sizes for each of those age groups were unspecified. Roush et al.'s (1995) normative compensated admittance data are based on a positive to negative air-pressure sweep, air-pressure pump speed of 150 daPa/s, and compensation at the positive tail. The commonly available air-pressure pump speed of 200 daPa/s is very close to the 150 daPa/s of Roush et al. (1995).
Because children in this age group (similar to the children in the 7-24 months age group) show large changes in acoustic reflex thresholds over time and because the data on the percentages of present IARs and on IAR thresholds with the 226 Hz and 1000 Hz probe tones in this age group are sparse, it is proposed that IARs should be obtained for both probe tones and for both the BBN and 2000 Hz activators (as suggested for the 7-24 months age group). The presence of an IAR at 100 dB HL for either probe tone with either activator should be considered as a pass result, whereas the absence of the IAR for both probe tones and activators should be considered as a fail result.
The 95th percentiles for EECV are based on the findings of deChicchis et al. (2000) for the 2-3 years age group as Roush et al. (1995) did not examine EECV. The deChicchis et al. (2000) tympanometric parameters were similar to those of Roush et al. (1995) except that the pump speed was 600/200 daPa/s.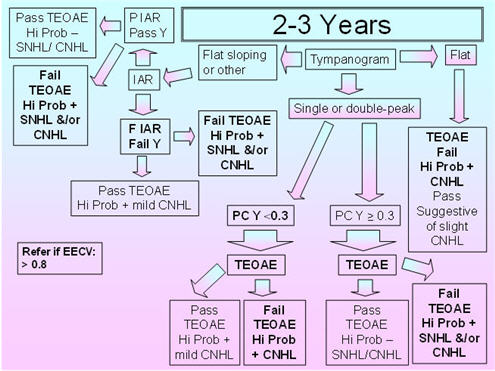
FIGURE 5. Proposed protocol for children between 2 and 3 years of age.
3 - 11 Years
Figure 6 shows the proposed protocol for children between 3 and 11 years of age. For the proposed protocol, immittance testing is based on the TPP as well as the IAR, compensated admittance, and EECV. The 226 Hz probe tone is used for IARs as well as for admittance tympanometric assessment. The normative data are based on the findings of Silman et al. (1992), who obtained sensitivity and specificity results for various acoustic immittance measures (individually and in combination) based on 53 ears of 53 participants (3-10 years of age) with normal-hearing sensitivity and normal middle-ear function and 82 ears of 54 participants (3-11 years of age) with middle-ear effusion based on pneumotoscopy and/or microtoscopy performed by an experienced otolaryngologist. Their tympanometric parameters included a positive to negative air-pressure sweep, the slower air-pressure pump speed of 50 daPa/s for more accurate determination of the TPP, and positive tail compensation. They used a TPP criterion of -100 daPa as the cutoff between normal and abnormal TPP based on the findings of Jerger (1970), Jerger, Jerger, and Mauldin (1972), and Jerger et al. (1974). Silman et al. (1992) used the findings from their control participants to calculate the 5th percentile for the compensated admittance. With this proposed protocol, the presence versus absence of the IAR for the 1000 Hz activator at 110 dB HL is determined, and testing at 110 dB HL is done if the IAR is absent at 100 dB HL. The normative EECV values are from Hanks and Rose (1993).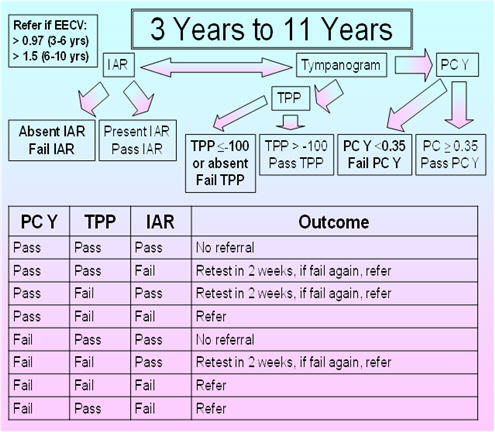
FIGURE 6. Proposed protocol for children between 3 and 11 years of age.
Silman et al.'s (1992) protocol, based on fail results for the tympanometric width and IAR, fail results for the compensated admittance and IAR, or fail results for the TPP and IAR, yielded a sensitivity of 90% and a specificity of 92.5%. In comparison, the ASHA (1990) protocol based on the tympanometric width and compensated admittance yielded a sensitivity of 81.5% and a specificity of 79%. In the group with middle-ear effusion having pure-tone air-conduction thresholds not exceeding 20 dB HL at 1000, 2000, and 4000 Hz, the sensitivity was 63% for the ASHA (1990) protocol versus 89.5% for the Silman et al. (1992) protocol. Tympanometric width has been excluded from the proposed protocol as considerable overlap on this measure has been observed between pathologic middle ears and normal middle ears. Furthermore, various middle-ear pathologies differentially affect (increase or decrease) the tympanometric width (Wiley & Fowler, 1994, p. 47). In the ASHA (1997) guidelines, the acoustic reflex was not included because of high over-referral rates noted in the ASHA (1990) guidelines. These over-referral rates had been associated with the use of 105 dB SPL as the criterion in the ASHA 1979 guidelines, but the ASHA (1979) and ASHA (1990) guidelines failed to consider the impact of maturation in the IAR criterion for pass versus fail results and the contribution of the multiplexing circuit in IAR measurement. These factors were accounted for by Silman et al. (1992) who employed a higher IAR criterion of 110 dB HL and measured the IAR using a device with a multiplexing circuit.
Adult acoustic immittance protocols can be applied to children who are about 11 or more years of age as acoustic-reflex thresholds approach adult values around that age (Jerger, 1970;Jerger et al., 1972;Osterhammel & Osterhammel, 1979). Unfortunately, with the advent of 1000 Hz admittance tympanometry, some manufacturers of immittance devices have withdrawn the capability to obtain susceptance and conductance tympanograms at 660 Hz. This prevents the application of the Vanhuyse, Creten, and Van Camp (1975) criteria for differentiation between ears with stiffening pathology and flaccid tympanic membranes, on the one hand, and ears with ossicular discontinuity on the other hand (Silman & Silverman, 1984). Numerous investigators have corroborated the association between the occurrence of abnormal 660 Hz B and G tympanograms (based on Vanhuyse et al., 1975) and ossicular discontinuity (Margolis & Popelka, 1977;Silman & Silverman, 1984;Van Camp, Creten, Vanpeperstraete, & Van de Heyning, 1980;Van de Heyning, Van Camp, Creten, & Vanpeperstraete, 1982)
Wideband reflectance is a promising, emerging technology for the assessment of middle-ear function. Several studies have demonstrated differences in mean wideband reflectance between individuals (especially and importantly for infants, including newborns) with and without middle-ear effusion, and case studies show differences in energy reflectance values between ears with and without middle-ear effusion (Feeney, Grant, & Marryott, 2003;Keefe et al., 2003, 2010;Keefe & Simmons, 2003;Margolis, Saley, & Keefe, 1999;Pisorski, Keefe, Simmons, & Gorga, 1999;Shahnaz, 2008). However, the sample sizes of such studies have generally been small, and sparse data are available on clinically feasible wideband reflectance criteria and procedures. Therefore, at this time, wideband reflectance should not be routinely employed in a clinical protocol for assessment of middle-ear function in infants and children.
In summary, although more studies are necessary to identify better ways to examine the variables associated with the pediatric ear, the protocols outlined in this paper provide clinicians with the information necessary to successfully test the middle ear status of children of all ages.
References
Abahazi, D.A., & Greenberg, H.J. (1977). Clinical acoustic reflex threshold measurements in infants. Journal of Speech and Hearing Disorders, 42, 515-519.
Alaerts, J., Luts, H., & Wouters, J. (2007). Evaluation of middle ear function in young children: Clinical guidelines for the use of 226- and 1,000 Hz tympanometry. Otology & Neurotology, 28, 727-732.
Alho, O.P., Oja, H., Koivu, M., & Sorri, M. (1995). Risk factors for chronic otitis media with effusion in infancy. Each acute otitis media episode induces a high but transient risk. Archives of Otolaryngology—Head & Neck Surgery, 121, 839-843.
American Speech-Language-Hearing Association. (1979). Guidelines for acoustic immittance screening of middle ear function. Asha, 21, 283-288.
American Speech-Language-Hearing Association. (1990). Guidelines for screening for hearing impairment and middle-ear disorders. Asha, 32(Suppl. 2), 17-24.
American Speech-Language-Hearing Association (1997). Guidelines for Audiologic Screening [Guidelines]. Available from www.asha.org/policy.
Anson, B.J., Bast, T.H., & Richany, S.F. (1955). The fetal and early postnatal development of the tympanic ring and related structures in man. Annals of Otology, Rhinology, and Laryngology, 64, 802-803.
Anson, B.J., & Donaldson, J.A. (1981). Surgical Anatomy of the Temporal Bone and Ear. Philadelphia: Saunders.
Baldwin, M. (2006). Choice of probe tone and classification of trace patterns in tympanometry undertaken in early infancy. International Journal of Audiology, 45, 417-427.
Bennett, M. (1984). Impedance concepts relating to the acoustic reflex. In S Silman (Ed.), The Acoustic Reflex: Basic Principles and Clinical Applications (Ch. 2, pp.35-61). New York: Academic Press.
Bennett, M.J., & Weatherby, L.A. (1982). Newborn acoustic reflexes to noise and pure-tone signals. Journal of Speech and Hearing Research, 25, 383-387.
Cantekin, E.I., Bluestone, C.D., Fria, T.J., Stool, S.E., Beery, Q.C., & Sabo, D.L. (1980). Identification of otitis media with effusion in children. Annals of Otology, Rhinology, and Laryngology (Supplement), 89, 190-195.
DeChicchis, A.R., Todd, N.W., & Nozza, R.J. (2000). Developmental changes in aural acoustic admittance measurements. Journal of the American Academy of Audiology 11, 97-102.
Driscoll, C., Kei, J., & McPherson, B. (2000). Transient evoked otoacoustic emissions in six year old school children: A normative study. Scandinavian Audiology, 29, 103-110.
Eby, T.L., & Nadol, J.B. (1986). Postnatal growth of the human temporal bone. Annals of Otology, Rhinology, and Laryngology, 95, 356-364.
Engel, J., Anteunis, L., Volovics, A., Hendriks, J., & Marres, E. (1999). Risk factors of otitis media with effusion during infancy. International Journal of Pediatric Otorhinolaryngology;48, 239-249.
Feeney, M.P., Grant, I.L., & Marryott, L.P. (2003). Wideband energy reflectance measures in adults with middle-ear disorders. Journal of Speech-Language-Hearing Research, 46, 901-911.
Hanks, W.D, & Rose, K.J. (1993). Middle ear resonance and acoustic immittance measures in children. Journal of Speech and Hearing Research, 36, 218-222.
Harris, F.P., & Probst, R. (2002). Otoacoustic emissions and audiometric outcomes. In MS Robinette & TJ Glattke (Eds.), Otoacoustic Emissions: Clinical Applications (pp. 213-242). 2nd ed. New York: Thieme
Himelfarb, M.Z., Popelka, G.R., & Shanon, E. (1979). Tympanometry in normal neonates. Journal of Speech and Hearing Research, 22, 179-191.
Holte, L., Cavanaugh, R.M. Jr, & Margolis, R.H. (1990). Ear canal wall mobility and tympanometric shape in young infants. Journal of Pediatrics,117(1 Pt 1), 77-80.
Holte, L., Cavanaugh, R.M. Jr, & Margolis, R.H. (1991). Developmental changes in multifrequency tympanograms. Audiology, 30, 1-24.
Jerger, J. (1970). Clinical experience with impedance audiometry. Archives of Otolaryngology, 92, 311-324.
Jerger, J., Jerger, S., & Mauldin, L. (1972). Studies in impedance audiometry. I. Normal and sensorineural ears. Archives of Otolaryngology, 96, 513-523.
Jerger, S., Jerger, J., Mauldin, L., & Segal, P. (1974). Studies in impedance audiometry II. Children less than six years old. Archives of Otolaryngology, 99, 1-9.
Joint Committee on Infant Hearing (JCIH). (2000). Year 2000 position statement: Principles and guidelines for early hearing detection and intervention program. American Journal of Audiology, 9, 9-29.
Keefe, D., Bulen, J.C., Arehart, K.H., & Burns, E.M. (1993). Ear-canal impedance and reflection coefficient in human infants and adults. Journal of the Acoustical Society of America, 94, 2617-2638.
Keefe, D.H., Fitzpatrick, D., Liu, Y.W., Sanford, C.A., & Gorga, M.P. (2010). Wideband acoustic-reflex test in a test battery to predict middle-ear dysfunction. Hearing Research, 263, 52-65.
Keefe, D.H., & Simmons, J.L. (2003). Energy transmittance predicts conductive hearing loss in older children and adults. Journal of the Acoustical Society of America, 114, 3217-3238.
Kei, J., Allison-Levick, J., Dockray, J., Harrys, R., Kirkegard, C., Wong, J.,... Tudehope, D. (2003). High-frequency (1000 Hz) tympanometry in normal neonates. Journal of the American Academy of Audiology;14, 20-28.
Kei, J., Mazlan, R., Hickson, L., Gavranich, J., & Linning, R. (2007). Measuring middle ear admittance in newborns using 1000 Hz tympanometry: A comparison of methodologies. Journal of the American Academy of Audiology, 18, 739-748.
Keith, R. (1973). Impedance audiometry with neonates. Archives of Otolarygology, 97, 465-467.
Kunov, H. (1977). The "eardrum artifact" in ipsilateral acoustic reflex measurements. Scandinavian Audiology, 6, 163-166.
Lutman, M.E. (1980). Real-ear calibration of ipsilateral acoustic reflex stimuli from five types of impedance meter. Scandinavian Audiology, 9, 137-145.
Lutman, M.E & Leis, B.R. (1980). Ipsilateral acoustic reflex artefacts measured in cadavers. Scandinavian Audiology, 9, 33-39.
Marchant, C., McMillan, P., Shurin, P., Johnson, C., Turczyk, V., Feinstein, J., & Panek, D.M. (1986). Objective diagnosis of otitis media in early infancy by tympanometry and ipsilateral acoustic reflexes. Journal of Pediatrics, 109, 590-595.
Margolis, R.H., Bass-Ringdahl, S.B., Hanks, W.D., Holte, L., & Zapala, D.A. (2003). Tympanometry in newborn infants—1kHz norms. Journal of the American Academy of Audiology 14, 383-392.
Margolis, R.H., & Hunter, L.L. (2000). Acoustic immittance measurements. In RJ Roeser, M. Valente, H Hosford-Dunn (Eds.), Audiology Diagnosis (Ch. 17, pp. 383-424). New York: Thieme.
Margolis, R.H., & Popelka, G.R. (1975). Static and dynamic acoustic impedance measurements in infant ears. Journal of Speech and Hearing Research, 18, 434-443.
Margolis, R.H., & Popelka, G.R. (1977). Interactions among tympanometric variables. Journal of Speech and Hearing Research, 20, 447-462.
Margolis, R.H., Saly, G.L., & Keefe, D.H. (1999). Wideband reflectance tympanometry. Journal of the Acoustical Society of America, 106, 265-280.
Margolis, R.H., & Shanks, J.E. (1990). Tympanometry: Basic principles and clinical applications (pp. 179-246). In WF Rintelmann (Ed.), Hearing Assessment. 2nd ed. Austin, TX: Pro-Ed.
Mazlan, R., Kei, J., Hickson, L., Stapleton, C., Grant, S., Lim, S.,...Gavranich, J. (2007). High frequency immittance findings: Newborn versus six-week-old infants. International Journal of Audiology, 46, 711-718.
Mazlan, R., Kei, J., & Hickson, L. (2009). Test-retest reliability of the acoustic stapedial reflex test in healthy neonates. Ear and Hearing, 30,295-103.
Meyer, S.E., Jardine, C.A., Deverson, W. (1997). Developmental changes in tympanometry: a case study. British Journal of Audiology, 31, 189-195.
Owens, J.J., McCoy, M.J., Lonsbury-Martin, B.C., & Martin, G.K. (1992). Influence of otitis media on evoked otoacoustic emissions in neonates during the first 24 hours of life. Seminars in Hearing, 13, 53-66.
Osterhammel, D., Osterhammel, P. (1979). Age and sex variations for the normal stapedial reflex thresholds and tympanometric compliance values. Scandinavian Audiology, 8, 153-158.
Palmu, A., Puhakka, H., Huhtala, H., Takala, A., & Kilpi, T. (2001). Normative values for tympanometry in 7- and 24-month-old-children. Audiology, 40, 178-184.
Paparella, M.M., Shea, D., Meyerhoff, W.L., & Goycoolea, M.V. (1980). Silent otitis media. Laryngoscope, 90, 1089-1098.
Paradise, J. (1980). Otitis media in infants and children. Pediatrics, 65, 917-943.
Paradise, J., Smith, C., & Bluestone, C. (1976). Tympanometric detection of middle ear effusion in infants and young children. Pediatrics, 58, 198-210.
Pisorski, P., Keefe, D.H., Simmons, J.L., & Gorga, M.P. (1999). Prediction of conductive hearing loss based on acoustic ear-canal response using a multivariate clinical decision theory. Journal of the Acoustical Society of America, 105, 1749-1764.
Rhodes, M., Margolis, R., Hirsch, J., & Napp, A. (1999). Hearing screening in the newborn intensive care nursery: Comparison of methods. Otolaryngology - Head and Neck Surgery, 120, 799-808.
Robertson, E.O., Peterson, J.L., & Lamb, L.E. (1968). Relative impedance measurements in young children. Archives of Otolaryngology, 88,162-168.
Roush, J., Bryant, K., Mundy, M., Zeisel, S., & Roberts, J. (1995). Developmental changes in static admittance and tympanometric width in infants and toddlers. Journal of the American Academy of Audiology, 6, 334-338.
Saunders, J.C., Kaltenbach, J.A., & Relkin, E.M. (1983). The structural and functional development of the outer and middle ear. In R Romand (Ed), Development of Auditory and Vestibular Systems (Ch. 2, pp. 27-46), New York: Academic Press.
Schwartz, D.M., & Schwartz, R.H. (1980). Acoustic immittance findings in acute otitis media. Annals of Otology, Rhinology, and Laryngology (Suppl), 89, 211-213.
Shahnaz, N. (2008). Wideband reflectance in neonatal intensive care units. Journal of the American Academy of Audiology, 19, 419-429.
Shahnaz, N., Miranda, T., & Polka, L. (2008). Multifrequency tympanometry in neonatal intensive care unit and well babies. Journal of the American Academy of Audiology, 19, 302-418.
Sells, J.P., Hurley, R.M., Morehouse, C.R., Douglas, J.E. (1997). Validity of the ipsilateral acoustic reflex as a screening parameter. Journal of the American Academy of Audiology, 8, 132-136.
Shanks, J. (1984). Tympanometry. Ear and Hearing, 5, 268-280.
Shurin, P.A., Pelton, S.I., Finkelstein, J. (1997). Tympanometry in the diagnosis of middle-ear effusion. New England Journal of Medicine, 290, 412-417.
Silman, S., & Silverman, C.A. (1984). Auditory Diagnosis: Principles and Applications (pp. 71-136). San Diego: Singular Publishing.
Silman, S., Silverman, C.A., & Arick, D. (1992). Acoustic-immittance screening for detection of middle-ear effusion in children. Journal of the American Academy of Audiology, 3, 262-268.
Sprague, B.H., Wiley, T.L., & Goldstein, R - Double- and single-peaked tympanometric shapes generally are associated with normal middle-ear function


