This study addresses measures and methods for identifying three under-recognized neuroreflexes of the external ear canal. These three neuro-reflexes are related to hearing instrument dispensing practice, as they often complicate and may cause failure to fit hearing instruments. By using an External Ear Neuro-reflex Checklist (EENC) clinical tasks such as video-otoscopy, cerumen management, otoblock insertion, ear-impression taking, and hearing aid adaptation can be more effectively managed. The EENC is a significant measurement tool and helps expose potential fitting problems during the evaluative process. Increased awareness of little-known ear dynamics can help reduce negative incidences and unnecessary remakes, modification and returns for credit.
Introduction:
The physiology of the human external ear canal poses significant challenges with respect to wearing and fitting hearing aids, wearing and fitting ear protection devices, using stethoscopes, and the application of various inserts for hearing and ear measurement techniques and many other functions. Modern ear and hearing devices require cooperation and accommodation of "pre-modern" natural defensive mechanisms designed to protect the external ear from foreign objects, infection, injury, invasion, and environmental exposure (Durrant and Lovrinic, 1984). Disregard of these same defensive mechanisms can cause discomfort or rejection of an otherwise beneficial or necessary auditory task.
The percentage of people who experience negative or perhaps unsatisfactory events or experiences during hearing loss evaluation and hearing aid exploration is significant. The Hearing Industries Association (HIA) statistics revealed a high rate of "return for credit" for hearing aids, hovering around 20% (Ross, 2002). Further evidence of dissatisfaction is apparent in the number of hearing aids "in the dresser drawer" (Kochkin, 2000). Additionally, shell and earmold remakes at hearing aid factories and earmold laboratories and countless in-office modifications made every day, indicate the presence of an underlying problem (Chartrand, 1999).
As a result of extensive clinical observation and a review of the current literature, three neuro-reflex mechanisms have been identified. These three neuro-reflexes affect hearing health practice procedures and hearing instrument success (Chartrand, 2004a).
For the purposes of this paper, these neuro-reflexes are referred to as:
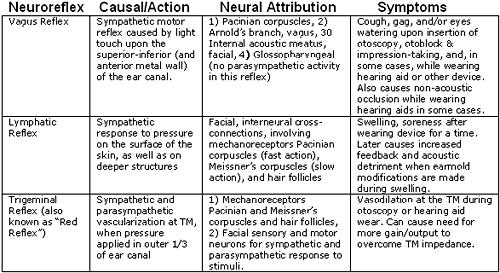
- The Vagus Reflex- This reflex may be evoked during insertion of the otoblock, cerumen removal, and rarely, with hearing aid use. This reflex is evidenced by cough, gag, and/or watering eyes during any of these activities.
- The Trigeminal Reflex is sometimes called the "red reflex," can cause excessive vascularization and thickening of the tympanic membrane (TM) during otoscopy, otoblock insertion, and during hearing aid wearing.
- The Lymphatic Reflex is a slower reflex which may result from over-wearing of hearing aids during the early adaptation period. This reflex is evidenced by excessive swelling of tissues and soreness while wearing a custom fitted earmold or hearing aid. Sometimes, even a perfectly fitted device (earmold or hearing aid) causes this reflex. The lymphatic response may appear to be due to an allergic reaction, or the result of a poor fit. Physical modification (buffing, drilling, grinding etc.) during the lymphatic response period potentially results in loose fittings, acoustic feedback and a compromise of the integrity of the amplification system -- acoustically and physically.
Neurologically, cranial, cervical and auxiliary nerves are implicated in the neurocomplex which innervates the external canal region. Although most of these have nothing to do with hearing, they impact the ear and surrounding tissue. The tympanic branch of the glossopharyngeal nerve (CN IX), the mandibular and maxillary branches of the trigeminal nerve (CN V), the mandibular, submandibular, chorda tympani, greater petrosal and tympanic plexus of the facial nerve (CN VII), Arnold's branch of the vagus nerve (CN X), and others, interconnect in a complex and difficult to predict (with regard to their impact on hearing prosthetics) manner (UC-Davis, 2005)
One perplexing challenge is adaptation to tactile pressure in the external ear canal. Elsewhere in the human body, adaptation to tactile pressure on a cutaneous sensory organ occurs only a short while after movement ceases, such as wearing a wristwatch or jewelry (Nafe and Wagoner, 1941). However, regarding prosthetics in the external ear canal, movement never ceases because of mandibular, facial, and other dynamics of the head and neck region. In fact, at the aperture of the ear, dimensional changes of 10% (or more) occur with the simple opening and closing of the mouth (Oliviera et al., 2005; Oliviera et al., 1992). Therefore, it is almost impossible for sensory receptors in the external ear to stop firing while a rigidly fixed object (i.e., hearing aid or earmold) is placed in an excessively movable ear canal (Kolpe and Oliviera, 2003). Aging ears can be even more adversely affected by these factors (Willott, 1981).
The degree of sensitivity varies widely in individuals, often depending upon the thickness of epithelium and the corneum stratum (keratin layer) over the surface of the external ear canal and tympanic membrane (Chartrand, 2004b; Kolpe and Oliviera, 2003; Naiberg, Proops, and Hawke, 1984). The most important factor regarding whether an individual will adapt comfortably to aural prosthetics may hinge on whether the keratin layer of their external ear tissue—which shields otherwise sensitive neuroreflexes as well as maintain physical homeostasis—is healthy and intact (Chartrand, 2004a).
To help organize these autonomic mechanisms into practical models that can be observed, understood, and accommodated (where possible) in the course of hearing health practice, each neuro-reflex has been defined in behavioral terms in Table 1 (below).
Table 1. Description of external ear neuro-reflexes affecting hearing instrument dispensing procedures and activities.
Method:
Participants-
Twenty-seven hearing impaired individuals (18 male, 9 female) between the ages of 49 and 87, with a mean age of 683 years, were studied during the course of the hearing aid fitting process No attempt was made to randomize participants. Participants were recruited by informed consent as they presented at three dispensing offices in southern Colorado and Northern New Mexico over a two-month period.
In many respects this subject population (n = 27) was representative of that found in the typical hearing health practice. For instance, there is a much higher concentration of serious hearing impairment in the older population, and the incidence of hearing impairment in males over sixty years of age is roughly twice that of females in the same age-group when comparing age and degree of hearing impairment (Staab, 1990).
Measures/Apparatus:
Equipment and materials used to make our observations consisted of the following:
American Electromedic AE-105 TympanometerA standardized checklist, the External Ear Neuro-reflex Checklist (EENC) with rating scale, was used to make qualitative measurements and observations of each task and their resulting reflexes (the EENC can be found in Appendix A).
Qualitone CD-3 Acoustic Appraiser
MedRx Video Otoscope
Welch-Allyn Hand-held Fiber-optic Otoscope
Fiber Ear Vision Ear Light Set
Westone Labs open-cell foam otoblocks
Dreve impression syringe
Dreve Oto-Form A/k silicone impression material
Cerumen management implements and solutions
Miracell Botanical Solution
Procedure:
Clinical tasks relative to assessing and evaluating hearing, and the hearing aid fitting process were performed. Careful observations (EENC) were recorded during; Video otoscopy, cerumen management, Oto-block insertion, impression material insertion & removal, earmold insertion, and post-fitting adaptation.
Table 2. EENC rating scale key.
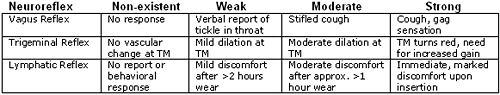
The Vagus Reflex (involving mostly Arnold's Branch) was rated according to patient reports and involuntary coughing, gagging, and/or eyes watering during any of the targeted clinical tasks. Trigeminal Reflex observations were made during and after certain tasks with a hand-held fiber-optic otoscope. Most of the responses for the Lymphatic Reflex were elicited during adaptation of new hearing aids or earmolds.
Data Analysis:Two sets of data were calculated from this study:To determine an association between "keratin status" and vagus sensitivity during otoblock insertion, we designated keratin status by number. Normal appearance was designated "0," thin or granulated appearance was assigned "1," a "peeling" or "peeled" appearance was assigned a value of "2'" and an appearance that was absent/swabbed was assigned a value of "3." Vagus sensitivity was quantified in reverse order (0 = non-existent, 1 = weak, 2 = moderate and 3 = strong).type="a">
- The Pearson Product Moment Correlation co-efficient between
keratin status and an elicited Vagus Reflex during otoblock insertion- Quantification of how often any of the targeted reflexes occur
at any point in the process.
Table 3. Ordered arrangement of subjects (n=27) re keratin status vs vagus reflex sensitivity.
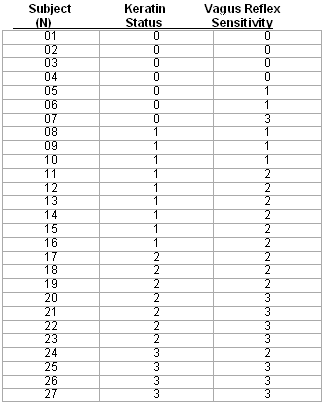
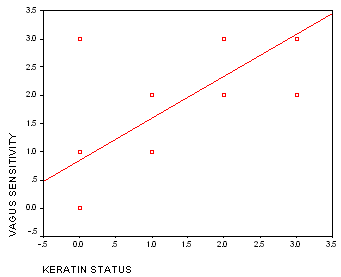
Exhibit B shows calculations for inferential and descriptive statistics from the above data. For a visual correlational representation of the data, a scatterplot with line of regression (r = 735) is shown in Figure 1:
Figure 1. Relationship between keratin status and vagus oversensitivity during otoblock insertion.
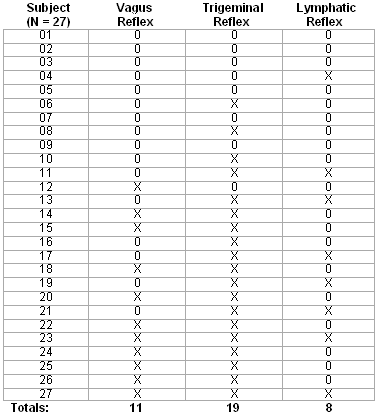
The second set of data concerned how often any of the three reflexes occurred in the subject (n = 27) population at any point in the evaluation, fitting, and post-fitting process. An "X" represents reflexes were observed (moderate or strong only), an "0" represents no significant reflex was observed.
Pass/fail decisions were based on the following criteria: Vagus if a cough reflex was evoked in at least two procedures, Trigeminal if a visible and rapid vascular dilation occurred during two or more procedures, and Lymphatic upon discomfort or soreness during the adaptation period. Data is tabulated in Table 4 as follows:
Table 4. Incidence of significant neuroreflex activity within the subject (n = 27) population.
Results/Discussion:
Keratin status was chosen as an important biomarker for external ear canal neuro-reflexes because it was the one physical characteristic that appeared to be an explanatory variable during most observations. We found a strong association (Pearson product moment correlation coefficient r = 735) between the protective status of keratin (corneum stratum) and the degree of sensitivity to stimuli on the surface of the external ear canal during otoblock insertion
In many cases, vagus sensitivity subsided as subjects were counseled to avoid practices and substances that are unhealthy for the external ear. By the end of the two month period, far fewer subjects experienced complaints related to a reflex of coughing, gagging, or watering eyes or non-acoustic occlusion
Regarding incidence of any reflexes among the subject population (n = 27), we generally found that those subjects who had poor keratin status also exhibited the greatest incidence of trigeminal-related symptoms. Of course, the trigeminal reflex is not necessarily an indicator of abnormality, except when over-vascularization of the TM creates sufficient impedance as to require extraordinarily additional amplification and output. Otherwise, eliciting a "red reflex" during otoscopy may be a sign of a normally functioning neurological system.
Surprisingly, there seemed to be no correlation between keratin status and post-fitting complaints due to lymphatic swelling. This may be because patient counseling relative to care of the ears during the early stages of the process masked what might have otherwise become problem cases. After cessation of use of cotton swabs in the ear canal, and other unadvisable practices, absent or thin keratin grew back sufficiently to reduce the incidence of swelling and soreness during adaptation to hearing aid use for some subjects.
From the data in Table 4, it was found that 41% of subjects exhibited a cough, gagging, and/or watering eyes during at least two of the procedures. The predominant activities exposing this sensitivity were cerumen management and otoblock insertion. A much larger (70%) percentage exhibited a significant trigeminal reflex during at least two procedures, usually during prolonged otoscopy and during impression taking.
27% of subjects experienced discomfort or tissue swelling during the first three days of adaptation to new hearing aids. It was determined, in the vast majority of these cases, that earmold fit was not the problem, but rather the dimensional variations that occur during mandibular movement and lack of careful observance of the recommended wearing schedule. Secondarily, it appeared that a failure to produce a protective layer of keratin in the ear canal and/or dermatitis response to otoplastics caused the majority of the remainder of such complaints. In two cases, oversensitivity in the lymphatic reflex prevented the wearing of conventional earmolds, which were remedied by open ear or tube fittings.
The information presented (above) can prove crucial in assisting hearing impaired patients to realize greater amplification and auditory rehabilitative success. The most important discovery of this study was the importance of observing and recognizing keratin status during the process of evaluating and fitting of hearing aids. The degree of sensitivity of defensive reflexes was largely determined by the amount and health of the keratin layer covering of external ear epithelium.
Hearing healthcare professionals may effectively assist hearing aid users in achieving greater hearing aid comfort by counseling users to:
- Use cotton-swabs no deeper than the aperture of the external ear canal, if at all
- Refrain from using ear solutions containing boric acid, hydrogen peroxide, or acetic acid on a frequent or routine basis. All of these chemicals remove keratin from the ear canal.
- Not attempt self-administered cerumen removal
- Adhere to a strategic and customized wearing schedule while adapting to new earmolds and hearing aids
 About the author...
About the author...Max Stanley Chartrand serves as Director of Research, DigiCare Hearing Research & Rehabilitation, Rye, CO, and is writes and lectures widely in the hearing health industry. He is also a full-time doctoral student at Northcentral University. Correspondence: www.digicare.org
View Appendix
References:
Carlson, N.R., (2004). Physiology of Behavior (8th edition). Boston, MA:Allyn & Bacon. ISBN 0-205-30840-6.
Chartrand, M.S., (2004a, July-August). The importance of the keratin layer to successful fitting and treatment. The Hearing Professional, 53(4):5-7, 31).
Chartrand, M.S., (2004b). Utilizing Neurophysiology in Resolving Hearing Aid Fitting Problems. Retrieved on July 16, 2004 from www.digicare.org
Chartrand, M.S., (2003, July 30). Diabetes Mellitus & Hearing Health 2003. AudiologyOnline, retrieved on January 27, 2005, from www.audiologyonline.com
Chartrand, M.S., (1999). Hearing Instrument Counseling: Practical Applications for Counseling the Hearing Impaired Livonia, MI:International Institute for Hearing Instruments Studies.
Durrant, J.D., and Lovrinic, J.H., (1984). Bases of Hearing Science, 2dn edition, Baltimore: Williams & Wilkins, pp. 248-250.
Feldman, A.S., "Acoustic Impendence-Admittance Battery", in Handbook of Clinical Audiology, 2nd edition, ed., Katz, J., Baltimore: Williams & Wilkins, pp. 356-374 (1981).
Grenness, M.J., (1999). Mapping morphologic change in the external ear. Masters dissertation, Hobart, Australia:University of Tasmania.
Gupta, D., Verma, S., and Vishwakarma, S.K., (1986). Surgical Radiological Anatomy, 8(4):217-220.
Hadjikoutis, S., Wiles, C.M., and Eccles, R., (1999). Cough in motor neuron disease: A review of Mechanisms. Quarterly Journal of Medicine, 92: 487-494.
Holder, L.E., (2001, June 20). Reflex Sympathetic Dystrophy. eMedicine, retrieved on January 27, 2005, from www.emedicine.com/radio/topics596.htm
Kolpe, W., and Oliviera, R. J., (2003). Chemistry and rheology of Otoplastic materials. In: Pirzanski, C., ed. Ear impressions and New Laser Shell Technology, Seminars in Hearing, 4(24):289-298.
Kochkin, S., (2000). Why my hearing aids are in the drawer: The consumer's perspective. The Hearing Journal, 53(2):34-42.
Kress, M., and Zeilhofer, H.U., (1999). Capsaicin, protons, and heat: New excitement about nociceptors. Trends in Pharmacological Science, 20:112-118.
Nafe, J.P., and Wagoner, K.S., (1941). The nature of pressure adaptation. Journal of General Psychology, 25:323-351.
Naiberg, J.B., Proops, D.W., and Hawke, M., (1984, April). Thickness of the migratory epithelium of the external auditory canal. Archives of Otolaryngology, 100(4):253-257.
Oliviera, R., Hammer, B., Stillman, A., Jons, C., Margolis, R., and Holm, J., (1992). A look at ear canal changes with jaw motion. Ear and Hearing, 13(6):464-466.
Oliviera, R., Babcock, M., Venem, M., Hoeker, G., Parish, B., and Vasant, K., (2005, February). The Dynamic Ear Canal and its Implications: The problem may be the ear, and not the impression. The Hearing Review, 12(2):18-19, 82.
Ross, M., (2002). The case for the trial period. Pennsylvania SHHH, retrieved on January 20, 2005, from www.pa-shhh.org/ross/ross40.html.
Spray, D.C., (1986). Cutaneous temperature receptors. Annual Review of Physiology, 48:625-638.
Staab, W.J., (1990, Fall). Hearing in the Elderly, Part I. Audecibel, pp. 8-13.
Surr, R.K., Cord, M.T., Walden, B.E., "Response of hearing aid wearers to the absence of a user-operated volume control", Hearing Journal, 54(4):32-36.
UC-Davis, (2005). General arrangement of the cranial nerves. Retrieved on January 25, 2005, from www.ucd.ie/ vetanat/ga-subject/head&neck/hn2.html.
Willott, J.F., (1981). Aging and the Auditory System: Anatomy, Physiology, and Psychophysics, San Diego: Singular Publishing Group, Inc.

