Editor’s Note: This text course is an edited transcript of an Oticon Medical live webinar on AudiologyOnline. Download supplemental course materials.
Learning Objectives
- After this course learners will be able to describe the advantages of the MIPS technique.
- After this course learners will be able to describe characteristics of the most suitable patients for the MIPS procedure.
- After this course learners will be able to list the steps of performing MIPS.
Introduction
Dr. Holmberg: Today’s course will focus on why we have developed Minimally Invasive Ponto Surgery (MIPS), how it is done, the preclinical and clinical results, as well as patient benefits.
We’ll start with indications and benefits of bone anchored hearing implants. Then, we will discuss the MIPS in detail, why it's done, review the new instrumentation and components, and show how surgery is performed. It may be helpful for you to explain this to patients that might be anxious about undergoing surgery. I will also go over results from the development work and clinical work we've been doing for the past two years, and I’ll save time for questions and answers at the end.
One of the key benefits of bone anchored hearing systems is that you can perform a preoperative evaluation. This is one of the few implants where, before surgery, you can get a good idea of the benefits you're going to have, by trying the device on a test band or a soft band.
Bone Anchored Hearing Systems – Overview & Candidacy
Who are potential patients and candidates for a bone anchored hearing system? The basic principle of a bone anchored hearing system is that rather than sound entering the ear as acoustic vibrations, sounds are converted to vibrations using a transducer that stimulates and vibrates the bone of the skull (Figure 1). These vibrations are then led directly to both cochlea where they are handled just like typical sound signals, and converted into electrical potentials in the auditory nerves. The whole skull transfers the sound waves into the inner ears and from there, the sound moves further along to the brainstem and to the cortex.
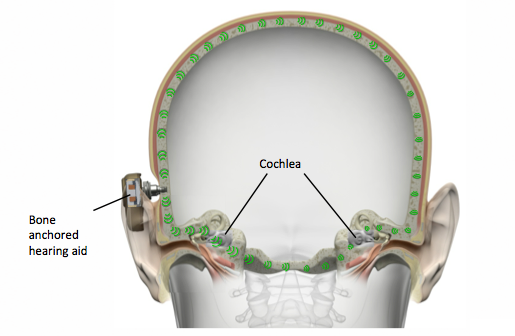
Figure 1. With a bone anchored hearing solution, sounds are converted to vibrations that stimulate the bones of the skull, and directed to both cochlea.
By vibrating the whole skull, we completely bypass the outer and middle ear. That means, the functioning of the outer and middle ear do not matter for a bone anchored hearing system. In terms of an audiogram, we consider only the bone conduction thresholds.
In terms of indications for a bone anchored hearing system, the primary indications are conductive or mixed hearing losses, and single-sided deafness (SSD). There are also a few other indications that we won't spend much time on today, but I will mention.
Typical audiograms for conductive hearing loss candidates have bone conduction thresholds close to normal, with an air bone gap. With mixed hearing losses, you also have a sensorineural component of the hearing loss. Typical diagnoses associated with these hearing losses are chronic otitis media, congenital malformation of the outer ear (also known as microtia), otosclerosis, and atresia.
Let’s take a closer look at a traditional hearing aid compared with a bone anchored hearing system for these patients (Figure 2). With the bone anchored hearing system, we only need to stimulate to reach the bone conduction thresholds, which are the inner ear thresholds. A traditional hearing aid will have to reach the air conduction thresholds, which includes the air bone gaps.
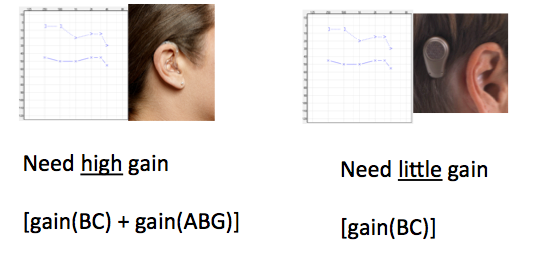
Figure 2. Gain requirements for a traditional hearing aid (left) versus a bone anchored hearing system (right) for the same audiogram.
The Ponto system, or any other bone anchored hearing system, does not need to compensate for the air bone gaps. With a traditional hearing aid, you need a high gain solution simply because you need enough gain to overcome the air bone gaps. When using a bone anchored solution to fit a conductive hearing loss, you need virtually no gain; you just need enough gain to vibrate the skull.
As you know if you work with hearing aids, high gain can cause challenges such as feedback and sound quality issues. Since the bone anchored system uses lower gain there is typically better sound quality for these patients and less problems with feedback. Hearing aids for conductive and mixed losses would require an earmold, possibly a tight-fitting one to prevent feedback. With a bone anchored hearing system, the ear canal is completely open.
When bone anchored systems were first introduced years ago, hearing aids had superior signal processing. Today, the technology in a Ponto system is just as advanced as that in a high-end modern hearing aid. The fitting software for Ponto is very similar to what is used for hearing aids, and it has basically the same programming capabilities.
Research indicates that when the air bone gap is larger than 30 dB, speech recognition is likely to be better with a bone anchored sound processor than with a traditional hearing aid (Mylanus et al., 1998; Wolf et al., 2010). That is a rule of thumb. If the air bone gap is around 25-30 dB, we recommended trying a bone anchored hearing system on a test band. You will likely see better outcomes with the bone anchored system as compared to the hearing aid.
In terms of fitting range, Oticon Medical offers two devices with two different maximum power outputs. We can fit up to an average bone conduction threshold of 55 dB.
With single-sided deafness, the bone anchored sound processor works like a CROS device (Figure 3). When you vibrate on the poorer hearing side, you will in fact vibrate the whole skull. The vibrations will also be carried over to the functioning cochlea, so the head, in a sense, functions like the wired or wireless link of a typical CROS device. The functioning cochlea will receive sound from both sides of the head – from the good hearing side via the typical path (ear canal, middle ear), and from the poorer hearing side transferred through the skull.
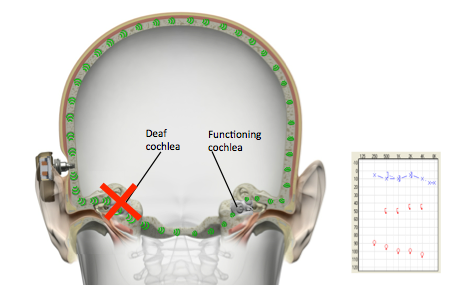
Figure 3. For SSD, the bone anchored hearing system works as a CROS device.
Single sided deafness, on the audiogram, refers to a profound sensorineural hearing loss in one ear, and normal hearing or near normal hearing thresholds on the better ear. The most common medical diagnosis with SSD is acoustic neuroma. Other diagnoses include sudden deafness, some congenital issues, some others that are less common.
In terms of fitting range, the average air conduction threshold on the better ear should be better than 20 dB HL.
In summary, candidacy for a bone anchored system consists of two primary groups – those with conductive or mixed hearing loss, and those with SSD. For conductive and mixed hearing loss, we can fit bone conduction thresholds up to an average of 55 dB HL. We know from research that if the air to bone gap is around 30 dB or larger, speech recognition will likely be better with a bone anchored hearing system than with a traditional hearing aid.
With single-sided deafness, the fitting range is normal hearing or near normal hearing on the better ear, and no further requirements for the poorer ear.
There is a third group, those with other medical issues. This group includes patients that have skin allergies in the ear canal or for other reasons cannot use an ear mold or a device in the ear canal. With a bone anchored hearing system, the ear canal is open, and can provide amplification without irritating or obstructing the ear canal.
Surgery
Over the years we have looked at ways to improve bone anchored implant surgery. A guiding star for us throughout the development has been to provide a truly minimally invasive technique because we strongly believe that less surgical trauma leads to better outcomes.
For example, in the past, we thought that thinning the skin around the implant and abutment was important to avoid skin reactions. We have learned over the last couple of years that in fact, it is better not to do any skin thinning at all in most cases. Rather, it is best to leave the healthy skin around the implant and the abutment. That, in turn, leads to better long-term outcomes.
The implant, or what is sometimes referred to as the fixture, sits in the bone, and integrates with the bone to provide a long-term stable connection to the skull. On top of that is the abutment, which is the part that comes through the skin, and it acts as an anchor point for the sound processor itself.
Our design features a very straight abutment. There is a very good match between the implant and the abutment, which has a straight neck through the skin. This design lends itself to a minimally invasive surgical technique involving a circular incision.
In our development of a more minimally invasive surgery, we developed a new surgical technique and new surgical components to protect the soft tissue during the surgery. This has led us to the idea of using a cannula, which I’ll explain in a moment.
Brief MIPS History
Before we move into how we actually perform the technique, let’s briefly look at some background and history. We started this project in June 2013. We had returned from the biggest conference on bone conduction hearing implants and hearing surgery, the Osseo Conference. We felt strongly that the minimally invasive techniques were leading to better long-term results, and were being quickly adopted by surgeons around the world.
Shortly thereafter, we performed the first preclinical installation of an implant to make a short feasibility study. Since then, we have been developing and testing hundreds of different versions of these instruments to get to a very user friendly and well thought through design. It's not something we have done on our own. We have been working with some of the world's leading surgeons, together with their patients. We involve these surgeons very early on in animal surgeries. In June 2014, the first surgeries were done with people, and to this day, they are happy users of the system.
In January 2015, we started running an evaluation of the MIPS technique across 15 centers in Europe. For regulatory purposes, we started testing in Europe. By June 2015, we received the feedback from the surgeries and the surgeons. I’ll share this with you later in the presentation, but virtually everyone felt this was a better technique than they were previously using, and wanted to continue with the minimally invasive technique. The last step in our process was creating a very user friendly kit pack system for surgeons.
How is MIPS Performed?
There are 5 steps of a MIPS surgery:
- Punch hold with a 5mm hole punch.
- Insert the cannula to protect the soft tissue throughout the surgery.
- Drill through the cannula.
- Remove the cannula, and insert the implant.
- Attach a healing cap and dressing.
Tailor Made Surgery
Cannula. If we take a closer look at the surgical components that we have developed, the centerpiece for doing this as minimally invasive and with as little trauma as possible is the cannula (Figure 4). The cannula has a number of functions. It is a hard stop that prevents the surgeon from drilling too deeply. Its key function is to protect the soft tissue during drilling. It also holds cooling fluid during drilling, which I will explain later. It also helps to guide direction to make sure that the implant is in the right direction.
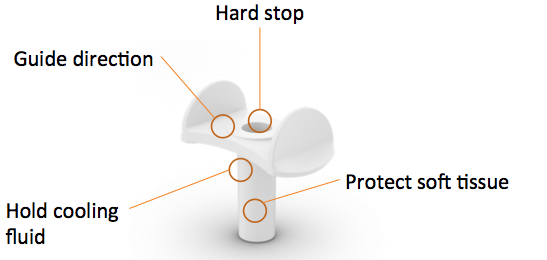
Figure 4. The cannula is the centerpiece of the MIPS surgical components.
Cannula drills. The drills are completely new and are a lot more advanced than they actually look (Figure 5). While they may look similar to drills you would see in a hardware store, I can assure you that they are not. The MIPS procedure requires a cannula guide drill, and a cannula widening drill.

Figure 5. MIPS cannula drills.
The drill includes a spacer that gives you different drill depths. The design of the drills also allows for removing of the hot bone chips, and cooling. In addition, they are coated with a low friction coating to reduce heat generation. They give a tactile guidance on performing the surgery at exactly the same spot over and over again. For those of you who are very familiar with these surgeries, I will add that we are still doing countersinking with these types of drills.
Implant installation is done with the insertion indicator (Figure 6). At this point in time, when you remove the cannula it is not easy to tell if the implant is fully inserted simply by looking. Instead, we use this insertion indicator that helps guide the surgeon in the right direction, optimizes alignment, and ensures full installation. The surgeon simply counts the number of turns the implant is engaging in the bone. By counting the right number of turns, which is four and a half turns for a 4mm implant, you know the implant was properly installed all the way down into position.
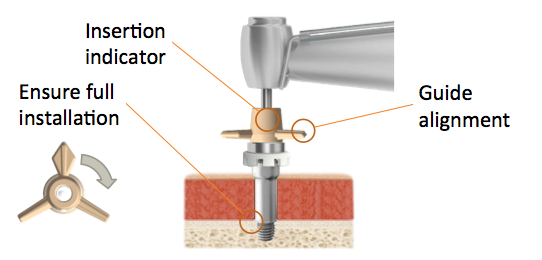
Figure 6. Implant installation includes an insertion indicator.
Finally, the soft healing cap is attached. It is made of a soft material, so it actually flips back rather than flips off if it gets a blow from the side. It also has an open interface, which is very handy for aftercare if, for instance, there is a soft tissue reaction. You can also use the soft healing cap together with the sound processor. If you have a little bit of skin reaction, you can now put on ointment for a week or so and still let the patient use the sound processor. This has proven very effective and is very well received by patients.
All of these MIPS components come packed in a kit, so you only have to open one thing in the operating room. In Figure 7, you can see the MIPS Surgery Kit 4 mm, which includes the cannula, the cannula guide drill, the cannula widening drill for a 4mm implant, and the soft healing cap. There is also a MIPS Back up Kit available for those who need to place a 3mm implant.
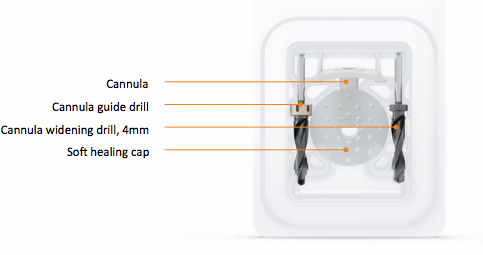
Figure 7. MIPS Surgery Kit 4mm
MIPS - Patient Selection
Minimally Invasive Ponto Surgery is a single-stage surgery. We reviewed the audiological inclusion criteria for a bone anchored hearing system. The MIPS technique is recommended for exactly the same patient groups as for current single-stage surgery. A single-stage surgery means that the implant and the abutment are placed during the same, single surgery. Those patients most typically are adults with normal bone quality and normal bone thickness. Children suitable for single-stage surgery are those with normal bone quality, and bone thickness estimated above 4mm. This means that they are typically 12 years old or older.
Finally, there is one additional criteria for this surgery: skin thickness cannot be thicker than 12mm. When skin thickness is thicker than 12mm, skin thinning is required, and then MIPS is not suitable. These issues will be managed by the surgeon, but it is good for audiologists to know for counseling purposes.
MIPS Step-by-Step
The following video is 9 minutes in length, and will take you step-by-step through the MIPS surgery.
![]()
MIPS step-by-step video (transcription follows in italics).
VIDEO narration: The long-term success of tissue preservation surgical techniques has inspired Oticon Medical to develop the Minimally Invasive Ponto Surgery, which is also referred to as MIPS. This video demonstrates the placement of a wide Ponto implant using the MIPS technique and its tailor-made MIPS surgical components.
MIPS is a single-staged procedure and is only recommended for patients suitable for a single-staged surgery. That is, primarily adult patients with normal bone quality and bone thickness of at least three millimeters, and where no complications during surgery are expected.
The sound processor indicator is useful to identify the best possible implant position, approximately 50-55 millimeters from the ear canal. Properly placed, the indicator must not touch the pinna or other personal assistive devices like the patient's glasses. Mark the implant position and shave the area.
The correct abutment length is critical for the long-term outcome. To choose the correct abutment length, measure the skin thickness using a thin needle and the Oticon Medical ruler. In this case, the 5mm skin thickness measurement suggests a 9mm abutment. During MIPS, usually local, sometimes general anesthesia is used. In this case, the surgeon injects local anesthesia. The area is cleaned and a sterile draping is placed.
An incision hole is made using a 5mm biopsy punch. Rotate the punch to incise the periosteum. Remove the periosteum at and around the implant site using a raspatorium. It is important that all periosteum and soft tissue is removed at the surgical site. The cannula should then be carefully inserted into the surgical site. Avoid creating tension in the skin as this will result in tension around the abutment.
There are few important aspects that are different when drilling with a cannula. First, please note that the cannula primarily acts as protection for soft tissue and is not a fixed position marker. In each drill step, ensure the following: That there is no soft tissue between the cannula and the bone; that the top of the cannula is parallel to the skin and that the cannula sits on the bone; that the cannula is filled with saline to facilitate cooling; continue to irrigate generously during the entire drilling procedure; and that the cannula drills are always inserted to the bone level before starting the drill. If you are drilling for the second or third time, use the tip of the drill to tactilely identify the previously drilled hole, that the cannula guide drill and cannula widening drill are always used together with the cannula. The cannula provides the stop that prevents drilling deeper than intended.
The recommended drill speed is 1,500-2,000 rounds per minute. Ensure the cannula guide drill is used with the spacer in place. Position the cannula with the top surface parallel to the skin and pressed against the bone. Fill the cannula with saline solution prior to drilling. Insert the cannula guide drill fully to the bone level. Begin drilling carefully applying irrigation to facilitate cooling. Stop drilling as soon as the spacer has reached the top of the cannula to avoid generating additional heat.
The bottom of the hole should then be checked carefully for bone using the double-ended dissector or another blunt instrument. If it is determined that sufficient bone thickness exists, remove the spacer from the guide drill. Consider the instructions for drilling with the cannula. Refill the cannula with saline solution. Insert the drill completely and feel to make sure the drill tip is placed in the previously drilled hole before starting to drill. Proceed with carefully drilling for a 4mm implant.
The cannula widening drill is used to widen the hole and prepare the bone for the implant. Maintain a drill speed of 1,500-2,000 rpm and select the appropriate widening drill, either 3mm or 4mm.
Consider the instructions for drilling with the cannula. Again, fill the cannula with saline solution and insert the drill through the cannula to the bone level. Use the drill tip to identify the previously drilled hole before starting the drill. Use generous irrigation. Carefully drill until the stop collar reaches the top of the cannula, then stop drilling. Flush the cannula and make sure any bone fragments are removed. Let the cannula stay in place to avoid contraction of the skin.
Set the drill unit to low speed with automatic torque control. For a 4mm long wide Ponto implant in normal quality adult bone, an initial torque setting of 40-50 Newton centimeters can be used. In softer bone, it is important to use lower torque setting to avoid damaging the drilled hole. Attach the insertion indicator on the abutment inserter and mount it on the handpiece. Pick up the implant with the premounted abutment. Remove the cannula from the surgical site.
Place the implant axially aligned in the drilled hole and start inserting the implant. It is important that the implant is inserted in line with the drilled hole and that it is fully inserted. While waiting for the preset torque to be reached, count of the number of turns that the implant engages in the bone before the drill unit stops. The number of turns can indicate if the implant is fully seated. As a guideline, a 4mm long implant should engage at least four and a half turns, and a 3mm long implant at least three and a half turns. Lift the handpiece straight up to release the abutment.
Attach the soft healing cap onto the abutment. In this case, ointment soaked ribbon gauze is wrapped around the abutment. Apply the dressing evenly and in appropriate quantities to create a light pressure against the skin to avoid hematoma but also to allow proper blood supply.
For further information on the procedure, please refer to the Oticon Medical surgical manual and the addendum including Minimally Invasive Ponto Surgery. MIPS can at any time be converted to a linear incision technique. In case of intraoperative complications, always consider converting to a linear incision for increased accessibility and visibility. All the components needed for perform MIPS are available in one convenient blister pack ,the MIPS Surgery Kit 4mm. There is also a MIPS Back up Kit, 3mm. Oticon Medical offers a complete set of non-disposable instruments.
Dr. Holmberg: This video and many other videos can be found on the Oticon Medical YouTube Channel. You can also order a hard copy from your Oticon Medical account representative.
MIPS Pre-Clinical Results
Let’s get into clinical results and outcomes in terms of user satisfaction. For more details, there is a whitepaper available on the Oticon Medical webpage and also on Research Gate.
New Drill System
There were three things we wanted to keep in mind throughout the development of the new drill system: avoiding heat-induced trauma that will kill off bone cells and cause implants to fall out; ensuring a good quality between the bone and implant surface for osseointegration and longevity of the implant; and being as atraumatic as possible. Sometimes during surgery, you do drill through the skull and into the dura, and in that case of course you want to be as atraumatic as possible.
Temperature
Regarding temperature, we have compared the temperature with our current system that has been used for thousands and thousands of implants with good outcomes, to the temperature with our new MIPS system.
We do this by adding a thermal coupler in artificial bone at a predefined distance from the drill, and then conducting measurements to determine what is happening with the temperature in the artificial bone. We chart those measurements on a graph.
We measure increases in temperature from normal body temperature, which is 37 degrees Celsius. First we used the guide drill, and then the widening drill, and we measured temperature increases from normal body temperature for MIPS with cooling and MIPS without cooling. We do this over and over and look at the maximum increase in temperature, and compare our current system with the new MIPS system.
Figure 8 shows the results of the tests for four scenarios. The first couple of tests were conducted without any cooling, and we saw a large increase in maximum average temperature, from 17 to 22 degrees Celsius. If you have ever used a drill to drill into any surface, you understand that drilling generates heat. With a heated drill and heated bone, you will have an increase in temperature.
If you don't cool with the current system, you will most likely lose the implant due to the high increase in temperature, which kills bone cells. The MIPS system is more efficient; it creates less heat than our current system. This is because we have the low friction coating, and the new twist drill design. However, we still need to cool properly and this is a very important point.
You can also see from Figure 8, that cooling significantly reduces temperatures in both systems. The average maximum increase with cooling is 3 to 4 degrees Celsius. When cooling is used, there is no difference between our current, well proven system and the MIPS system. We also tested cases with MIPS where the cannula was at a 45 degree angle and cooling was done, and again found no significant differences in temperature increase from our current system with cooling.
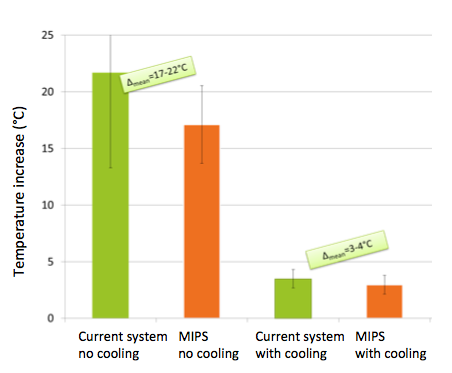
Figure 8. Temperature tests, MIPS compared with current procedure. Cooling significantly reduces temperatures in both systems.
In summary, the temperature increase with MIPS is similar to the current system when cooling is done. It is important that with both the current system and the MIPS system that during surgery the surgeon cools as instructed.
Drill efficiency
When we compare histological images of drill sites in bone using the current drill system and those with the new MIPS drills, we see a much smoother interface with the MIPS drills. The MIPS drilling protocol provided a clean cut edge of the bone.
If you're a surgeon, you will very clearly notice a difference in pressure with the new MIPS drills. Much less force is needed with the new drills, which surgeons appreciate. For patients, this means that drilling goes much faster. Previously, it took several seconds, and currently takes less than a second with the new MIPS system. If you have ever heard a dental drill, you know how loud and annoying drills can be for patients. The new MIPS system lessens that annoyance to patients during surgery.
An important safety aspect to consider is, what happens if the surgeon does drill through the bone completely to the dura. We have looked at this in the pig model, and what we found is that we do not penetrate the dura even when drilling 4mm deeper than the bone itself. There is no difference from this important safety aspect for the current system compared to the new MIPS system.
MIPS Clinical Results
We have been conducting a large multicenter randomized controlled trial since 2014. We are almost closing recruitment, and later in 2016, we will be able to give you more detailed results from this study.
While we don't have those results yet, I will share results of an evaluation we conducted across 15 centers and six countries in Europe. The design of this evaluation is a case series where we included adult patients eligible for single-staged bone anchored surgery, and we did follow up according to local preclinical practice.
Twenty-one surgeons were included across those 15 centers. The majority of the surgeons were relatively experienced, meaning they are doing at least 10 surgeries per year, but a few were doing considerably lower numbers of surgeries. So there was a range of experience among the surgeons. In terms of surgical technique used, 90% of the surgeons had adopted a tissue preservation technique, or techniques without skin thinning that we now recommend. They are using state of the art techniques with small differences. We compared our MIPS technique to modern tissue preservation type of techniques.
Intra-operative results
In 97% of the cases, the MIPS surgery was performed as instructed and as intended. In two cases, the surgeon decided to open up a small incision around the circular incision during surgery, and that is something that we always recommend as a backup solution if you feel uncertain at some point during the surgery.
Intra-surgical complications
In terms of intra-surgical complications, the numbers and issues were the same as what we typically see in any type of bone anchored surgery. In 73% of cases there were no complications. In a few cases, there was drilling into a vein. In a few cases, the dura was exposed, and outside of the trial it was reported that one case had a minor cerebrospinal fluid (CSF) leak that was closed with the implant itself. Again, that is something that is reported in approximately one percent of surgeries and nothing surprising. Overall, the intraoperative results were as we hoped and with the normal type of minor complications that we see with any bone anchored surgery.
Surgery time
In terms of surgery time, we found that surgical time decreases with experience. The first couple of surgeries take on average 20 minutes, but then it goes down to an average time of about 10 minutes with more experience, after five surgeries.
The majority of these surgeries are done under local anesthetics. It's a relatively fast, minimally invasive technique that you can do under local anesthetics for a vast majority of the patients.
Feedback from Surgeons
Figure 9 includes photographs from five typical patients one-week post surgery. These are the first five patients from the randomized control trial; we did not cherry pick the best patients to highlight. This is what the patients typically look like one week postoperatively. As you see from these images, they are almost perfectly healed at that point in time, and the hair is already starting to come back.
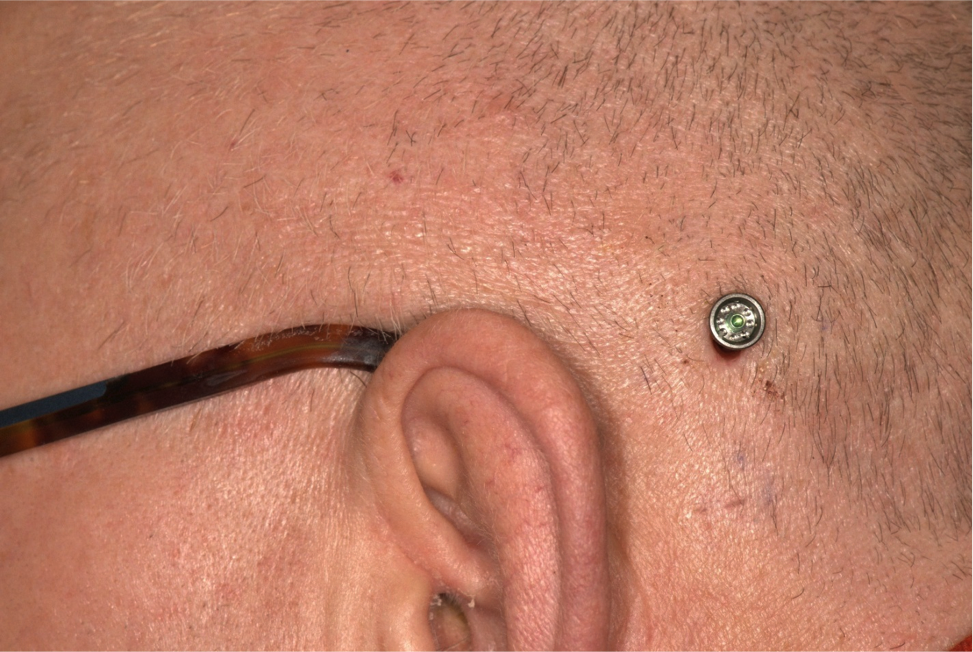
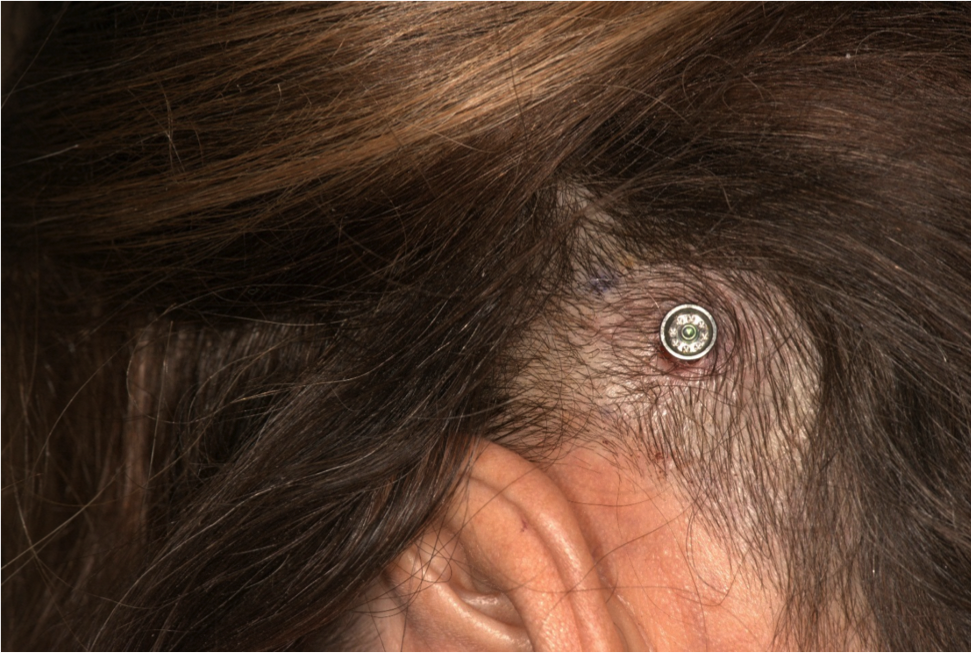
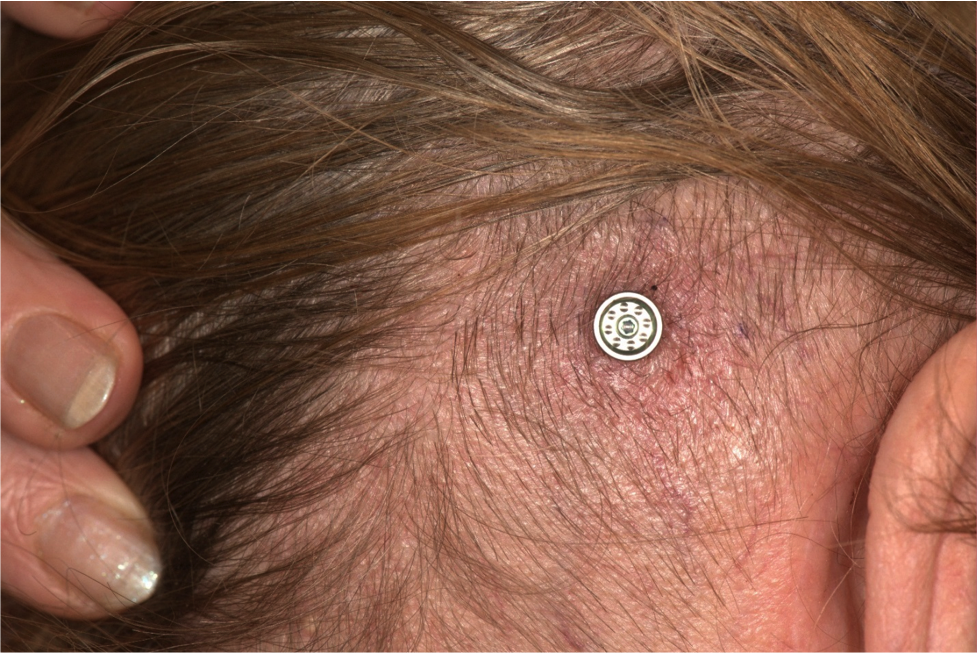
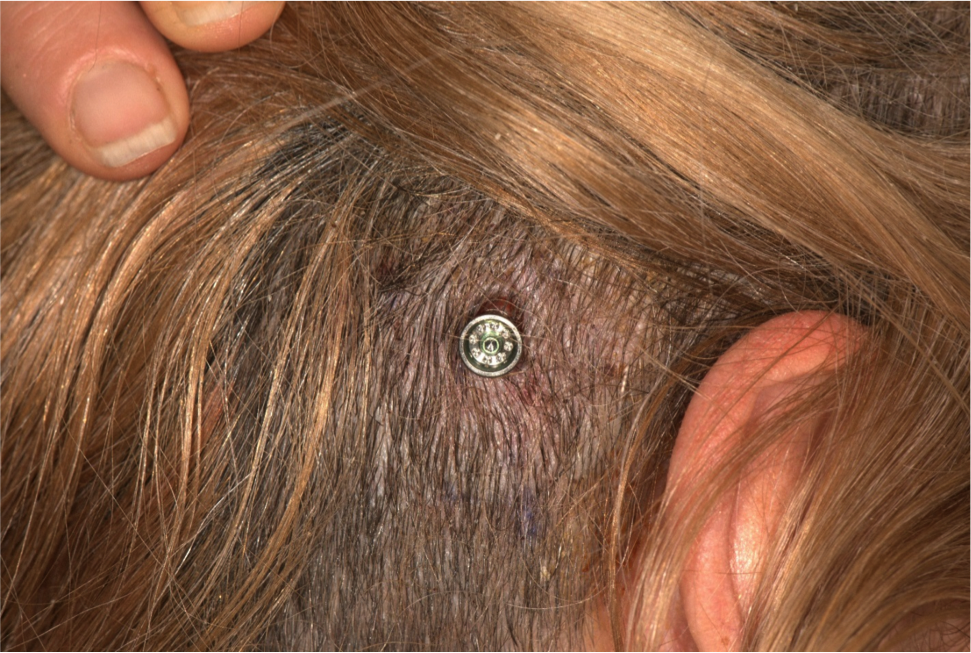
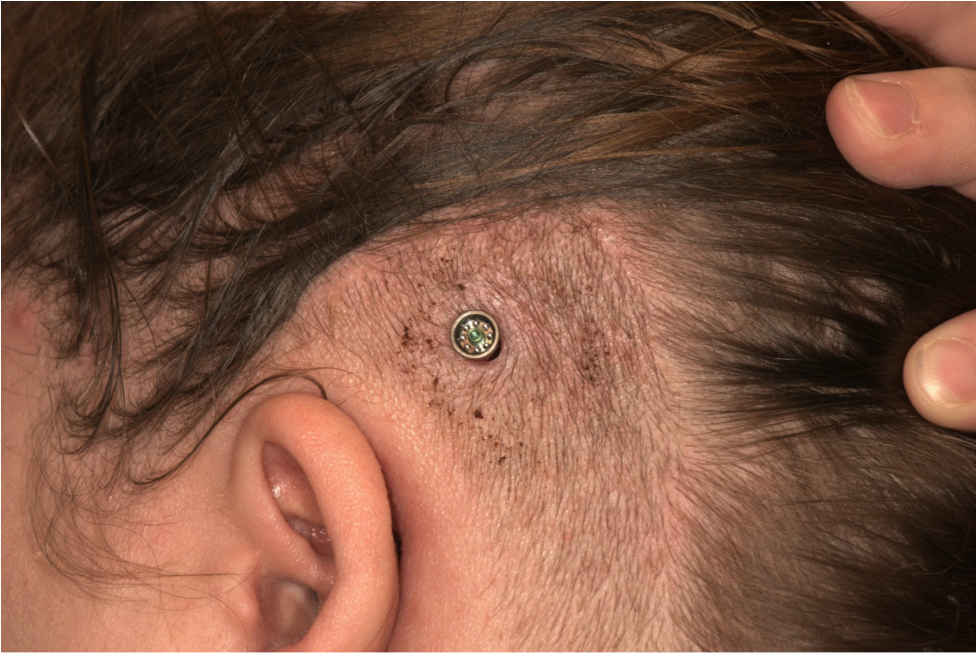
Figure 9. Five patients, one week post surgery.
There is no scarring. There are no sutures during the surgery that you need to remove. Patients come back for a quick follow-up to the ENT surgeon, and then once osseointegration has taken place, you can fit the sound processor.
We asked the surgeons a number of questions about their experience. One question was, “After how many MIPS cases did you feel confident/comfortable with the technique and instruments?”. About half of the surgeons reported that they needed one or two surgeries to be confident and comfortable with the new technique, while 33% said they were confident/comfortable right away. The new technique is different, and that's why we at Oticon Medical have said that we will train everyone in how exactly to do the MIPS technique before they start using it.
We also asked, “Are you likely to continue to use the MIPS procedure?”. Every surgeon except one wanted to continue using the MIPS technique.
As a company, we pride ourselves on putting patients first. We were very happy with the response of the next question, “Using MIPS, do you feel that you help your patients in a better way as compared to the previous technique?”. Remember, these surgeons were already using state of the art tissue preservation techniques with good outcomes. More than 85% of the surgeons felt that they helped their patients in a much better, or slightly better way with MIPS, and no one felt that they helped less or worse.
Clinical Results Summary
To summarize these clinical results, we find there is a learning curve for the surgeon. The current technique is primarily visual, while MIPS is a primarily tactile surgery. However, you have seen in the videos that we have designed the instruments to provide that tactile feedback during surgery.
The clinical results are very encouraging with few intraoperative complications, excellent healing and a good starting point for long-term successful implants. We have patient testimonials talking about how pleasantly surprised they were by the process. They went into surgery feeling anxious, were done in less than 15 minutes under local anesthetics, and were back home within a couple of hours. Of course, most patients have nothing to compare it to, but they are delighted that the surgery is quick and minimally invasive.
The surgeons are happy with the MIPS technique and feel they have helped their patients in a better way. I'm sure that those of you who are audiologists will be happy to see these patients coming into your clinic with the scarless result after the surgery.
I would like to acknowledge the centers we worked with for these clinical evaluations; they are listed in Figure 10.
Figure 10. Clinical evaluation centers.
Summary
In summary, bone anchored implants are indicated for mixed and conductive hearing loss, single sided deafness and a few other less common medical conditions.
Bone anchored systems completely bypass the ear canal and the middle ear. They provide a better sound quality for those patients that have an air bone gap larger than 30 dB.
MIPS, Minimally Invasive Ponto Surgery, is an updated and improved way of placing the bone anchored hearing implants. You can, of course, place the Ponto implant with other surgical techniques, but from now on we recommend the MIPS technique.
MIPS is meant for what we call single-stage patients, where you place the implant and the abutment during the same surgery. That's typically your adult patients with normal bone quality where you expect no complications during surgery, and older children, generally 12 years of age and older.
The MIPS technique is designed for creating minimal trauma during surgery. The whole instrumentation set was redesigned for as little trauma as possible, with the centerpiece being the cannula that protects the soft tissue during drilling. After placing the implant, there is no need for sutures, and there is virtually no scar tissue around the abutment. Thank you for your time and attention.
Questions and Answers
Regarding temperature, is there a difference between MIPS and traditional surgeries, and if so, is it significant?
Dr. Holmberg: Cooling is required for both the MIPS surgery and the traditional surgery. If you do cooling according to instructions, the temperature increase is the same with both MIPS and the traditional process.
However, we conducted tests without cooling in the laboratory using artificial bone, as I described, as we wanted to compare the two techniques to see if the MIPS drills themselves were more efficient. In this case, the MIPS system creates a lower temperature, which shows that the MIPS drills are indeed themselves more efficient and create less heat.
In actual surgeries where you do cooling as necessary and recommended, you end up at the same place in terms of temperature with MIPS and with the current system. I hope that answers your question.
Why are the MIPS drills more effective – is it because of the material they are made of?
Dr. Holmberg: Better efficiency is achieved mainly by using a twist design type of drill. The exact cutting angles of the tips of the drills create more efficient drills. The material of the drill itself does not influence the efficiency, but we do add a diamond-like carbon coating on top. That's why the drills look black, and that coating further reduces friction and therefore helps reduce heat generation.
References
For a full list of references, please refer to the course handout.
Mylanus et al. Intraindividual comparison of the bone-anchored hearing aid and air-conduction hearing aids. Otolaryngology- Head & Neck surgery. 1998; 124(3): 271-6
Wolf et al . Better performance with bone-anchored hearing aid than acoustic devices in patients with severe air-bone gab. The Laryngoscope 121:613-616, 2010.
Cite this Content as:
Holmberg, M. (2016, February). Minimally Invasive Ponto Surgery: A new perspective on bone anchored surgery. AudiologyOnline, Article 16235. Retrieved from https://www.audiologyonline.com

