 92 percent of clinical trial participants report improvement hearing in background noise in the first 12 months.
92 percent of clinical trial participants report improvement hearing in background noise in the first 12 months.
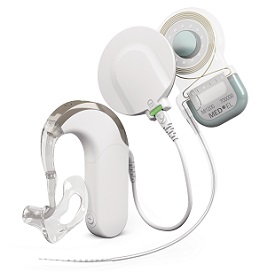
September 16, 2016 – (Durham, NC) – MED-EL USA announced today the FDA approval of the SYNCHRONY EAS (Electric Acoustic Stimulation) Hearing Implant System. EAS represents the latest innovation in hearing implants, and is the combination of two technologies: the revolutionary SYNCHRONY cochlear implant that stimulates the auditory nerve for high-frequency hearing loss, and the SONNET EAS audio processor, with built-in acoustic amplification for low-frequency hearing loss.
The FDA approval of the EAS System represents a major advancement for people who live with high-frequency hearing loss, but with existing low-frequency hearing levels that they are afraid to lose. This is often referred to as a “ski-slope” hearing loss based on the steeply sloping image represented on a candidate’s hearing test result. People with this type of hearing loss often have difficulty understanding speech in background noise with their hearing aids.
Ninety-seven (97) percent of patients participating in the MED-EL EAS clinical trial reported a benefit from EAS within the first 12 months. The study showed that 92 percent of participants reported an improvement in their ability to hear in background noise, one of the most challenging listening environments for people with hearing loss, and 90 percent reported satisfaction with the device overall. Additionally, 97 percent of patients were able to use the acoustic unit built into the audio processor, which can be enabled if patients have low-frequency hearing levels (80 dB or better) remaining after surgery. On average, patients performed more than twice as well on tests of speech understanding with EAS than they did with their hearing aids preoperatively.
“The EAS System has the potential to close the gap for people who have high-frequency hearing loss, but whose residual low-frequency hearing would have made them ineligible for a cochlear implant up until now,” said Raymond Gamble, CEO and President, MED-EL North America. “If you struggle with hearing in background noise with hearing aids, you may be a candidate for the EAS System.”
“Our clinical trial participants were overwhelmingly receptive to this new technology,” said Harold C. Pillsbury, MD, Chair, Department of Otolaryngology/Head and Neck Surgery, University of North Carolina School of Medicine. UNC participated in the EAS System clinical trials and implanted more patients than any other center. “Historically, it’s been a challenge to help people whose hearing has been ‘too good’ for cochlear implants, but who gain little to no benefit from hearing aids despite having low-frequency hearing. The EAS system fills that unmet need,” he added.
The EAS System is approved for candidates age 18 years and older who have normal to moderate sensorineural hearing loss in the low frequencies, sloping to a severe-to-profound hearing loss in the high frequencies. Single-word speech understanding scores are less than 60 percent in both ears preoperatively.
MED-EL expects that the SYNCHRONY EAS Hearing Implant System will be available in the coming months. Product availability updates will be posted on www.medel.com.
Hearing Loss in America
Hearing loss is a major public health issue in the United States. The National Institute on Deafness and Other Communication Disorders estimates that 15 percent of Americans (26 million people) between the ages of 20 and 69 have high-frequency hearing loss due to exposure to noise at work or during leisure activities. Hearing loss is also associated with age. While 2 percent of adults aged 45 to 54 have disabling hearing loss, the rate increases to 8.5 percent for adults aged 55 to 64. Nearly 25 percent of those aged 65 to 74 and 50 percent of those who are 75 and older have disabling hearing loss.
About MED-EL
Austria-based MED-EL Medical Electronics is a leading provider of hearing implant systems with 29 subsidiaries worldwide. The family-owned business is one of the pioneers in the industry. The two Austrian scientists Ingeborg and Erwin Hochmair developed the world’s first microelectronic-multichannel cochlear implant, now considered the modern cochlear implant, which was implanted in 1977. The cochlear implant was and remains the first replacement of a human sense, the sense of hearing. In 1990 the Hochmairs laid the foundation for the successful growth of the company when they hired their first employees. To date, the company has grown to more than 1,700 employees around the world.
MED-EL offers the widest range of implantable solutions worldwide to treat various degrees of hearing loss, including cochlear and middle ear implant systems, and the EAS (combined Electric Acoustic Stimulation) hearing implant system. In July 2016, MED-EL acquired the technology for a novel non-surgical bone conduction system from the Swedish medical device company Otorix, further expanding the number of people who can benefit from innovative hearing technology and reinforcing MED-EL’s mission to overcome hearing loss as a barrier to communication. People in over 100 countries enjoy the gift of hearing with the help of a product from MED-EL. Learn more at www.medel.com or the MED-EL Expo Page on AudiologyOnline.

