One of the key features of vestibular nystagmus is that in normal individuals, the nystagmus intensity is strongly reduced by visual fixation. Some patients however, are unable to sufficiently suppress vestibular nystagmus. In the standard electronystagmography (ENG) or videonystagmography (VNG) test battery, the patient's ability to suppress vestibular nystagmus is routinely evaluated during the caloric test. This article describes the proper procedure for administering the fixation suppression test and analyzing the results. The interpretation of the test and its relationship to the tracking and optokinetic tests are also discussed.
Procedure
The most common protocol for testing fixation suppression is based on the procedure described by Alpert (1974). During caloric irrigations, shortly after the peak of the response, the patient is instructed to fixate on a small stationary target. Because in ENG, caloric nystagmus is usually recorded with eyes closed, fixation can be induced by simply asking the patient to open his or her eyes and to look at a target straight ahead. In VNG, the fixation test can be done by removing the cover of the goggles and again asking the patient to look at a visual target. Alternatively, some VNG systems have a built-in LED light in the goggles that is controlled by the computer. During the fixation suppression test, the light can be turned on for visual fixation.
The following issues must be considered for optimal testing of fixation suppression:
- The best time to initiate visual fixation is shortly after the caloric response has reached its peak and the nystagmus intensity is beginning to decline. If the patient fixates before that, the actual caloric peak, which is the most important parameter in calculating unilateral weakness and directional preponderance, may be missed. If the patient fixates long after the response reaches its peak, the nystagmus intensity may not be sufficient to produce a valid test of fixation. Caloric responses usually reach their peak approximately 30 seconds after the irrigation ends (60-90 seconds from the onset of the irrigation depending on whether water or air irrigations are used). Therefore, it is best to ask the patient to fixate approximately 40-45 seconds after the end of the caloric irrigation. This will ensure that the nystagmus intensity just before fixation is still strong enough to produce a valid test of fixation suppression while at the same time, preserving the peak of caloric responses.
- The common protocol requires testing fixation suppression for at least one right-beating and one left-beating caloric response. Most laboratories perform the fixation test only during two caloric irrigations of the same temperature (usually warm irrigations). However, for valid comparison of fixation suppression for different nystagmus directions, the intensity of the nystagmus just before fixation should be approximately the same for two irrigations. If the patient has a significant unilateral weakness or directional preponderance, the pre-fixation nystagmus intensities for the same-temperature irrigations are likely to be substantially different for different nystagmus directions. This problem can be avoided by testing fixation in all four irrigations. The examiner can then select two irrigations, one for each nystagmus direction, where the nystagmus intensities just before fixation are strong and approximately equal.
- The tracings should be marked at the onset of fixation after making sure that the patient is actually attempting to fixate on a small visual target. Sometimes caloric irrigations cause dizziness and are distressing to the patient. In those cases, the patient may fail to follow the instructions to fixate. The examiner can sometimes recognize this by observing the patient's eyes during visual fixation. If the patient keeps his or her eyes closed or avoids looking directly at the target, the fixation test is probably invalid and the tracings should not be misidentified as failure of fixation suppression.
- After approximately 10-15 seconds, the fixation should be eliminated and the recording should continue while alerting the patient. Again, the tracings should be marked to identify the end of fixation.
The most common method for analyzing the fixation suppression test is to compare the nystagmus intensity after fixation with the nystagmus intensity before fixation (ANSI, 1999).
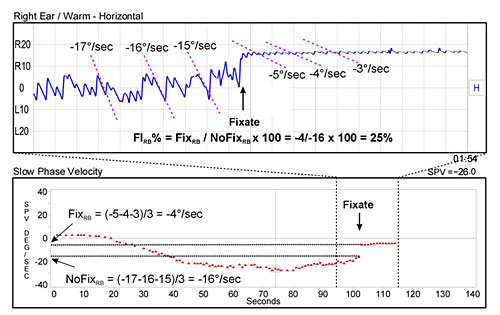
Figure 1. Analysis of the Fixation Suppression Test
Figure 1 shows an example of the following procedure:
- A 5-second time period just before fixation and a 5-second period just after fixation are identified. The slow-phase velocities of 3 typical nystagmus beats are calculated for each time period. If the nystagmus completely disappears after fixation, its intensity should be considered to be zero.
- It is best to avoid nystagmus beats that occur within 1 second before and 1 second after fixation. The nystagmus beats during the transitional period are usually contaminated by artifact and may cause error in estimating the intensities.
- The slow-phase velocities in each time period are averaged to determine the intensity of nystagmus for both before and after fixation conditions.
- The fixation suppression test is quantified by calculating a Fixation Index:
FI% = Fix / NoFix * 100
Where Fix is the nystagmus intensity for the time period after fixation, NoFix is the nystagmus intensity for the time period before fixation, and FI% is the fixation index expressed as a percentage. - If nystagmus is fully suppressed by fixation, FI will be 0%. If nystagmus is partially suppressed by fixation. FI will be between 0% and 100%. If nystagmus is enhanced by fixation, FI will be greater than 100%.
- The above procedure should be repeated for each direction of nystagmus to derive FI RB% for right-beating nystagmus and FI LB% for left-beating nystagmus.
There is disagreement on what values for the fixation index should be considered abnormal. Alpert (1974) observed that the normal limit for FI% was between 60% and 70%. Other reports have suggested a normal limit of 50% or lower (Demanez and Ledoux, 1970). Today, most laboratories consider FI% of less than 60% to be normal (Jacobson, Newman, and Peterson, 1993). As will be discussed in the limitations of the fixation suppression test, the ability to suppress vestibular nystagmus is age-dependent. Since we are not currently using an age-adjusted fixation index, perhaps any value between 50-60% is acceptable as long as the FI% values close to the normal limit are interpreted cautiously.
Interpretation
The fixation suppression test is a test of the visual-vestibular interaction. Patients with normal vestibular and oculomotor functions usually have normal caloric responses (i.e., approximately equal and symmetrical caloric peaks) and normal fixation suppression. Patients with unilateral lesions confined to the labyrinth or the vestibular nerve usually have asymmetric caloric responses (unilateral weakness and/or directional preponderance) but normal fixation suppression. Fixation suppression testing in patients with bilateral lesions of the labyrinth or the vestibular nerve is not possible because these patients do not generate adequate nystagmus intensities for a valid fixation test. Finally, patients with normal vestibular function but abnormal oculomotor function often have failure of fixation suppression. The caloric responses in these patients can be either normal or abnormal depending on the type of oculomotor lesion.
There is general agreement that failure of fixation suppression indicates a central lesion (Jacobson et al., 1993). The lesion can originate from the parietal-occipital cortex, the pons, or the cerebellum. However, failure of fixation suppression is most prominent in lesions involving the midline cerebellum (Baloh and Honrubia, 1990).
Fixation Suppression and Smooth Pursuit
The neural pathways responsible for suppressing vestibular nystagmus are closely associated with the smooth pursuit system. The pursuit system enables us to track small moving targets by maintaining the image of the target on the fovea, which is the most sensitive part of retina. When viewing a moving target, the image of the target moves across the fovea and retina and causes retinal (foveal) slip. This is the signal to the pursuit system to move the eyes in such a way that would minimize the retinal slip and keep the image of the target on the fovea. Normal tracking function depends on several conditions. First, the image of the target has to be small enough to fit on the fovea. If the image of the target extends beyond the fovea and covers parts of the retina, the resulting eye movements will be a combination of smooth pursuit and optokinetic responses. Second, the velocity of the target must not exceed the upper limit of tracking eye movements. The smooth pursuit system does not function well for velocities greater than 100 degrees per second or frequencies over 1 Hz. Third, the subject must choose to track a moving target because the pursuit function is under voluntary control. Finally, the pursuit pathways in the central nervous system must be intact.
During fixation suppression, the slow phase of vestibular nystagmus moves the image of a stationary target across the retina and generates retinal slip. It is reasonable to assume that the pursuit system will react by attempting to move the eyes in the opposite direction of the nystagmus slow phases. The suppression or complete abolition of nystagmus can be explained by the pursuit system preventing the vestibular system from moving the eyes away from a head-fixed target.
Clinical and experimental observations have established a strong association between failure of fixation suppression and smooth pursuit abnormalities in the tracking test. For example, Halmagyi and Gresty (1979) have demonstrated that patients with impaired fixation suppression consistently have abnormal smooth pursuit in the opposite direction of impaired fixation suppression. That is, for patients with abnormal tracking for rightward target movements, fixation suppression for left-beating caloric nystagmus is abnormal and vice versa.
Based on these observations, some investigators have suggested that the fixation suppression test is a test of the smooth pursuit system and nothing more (Barnes, Benson, and Prior, 1978). If so, the fixation suppression test is redundant because the pursuit function is tested in two other tests within the ENG test battery: the tracking and optokinetic tests. Note that the current laboratory method for testing the optokinetic system is in fact, another test of the smooth pursuit system.
The role of the pursuit system as the sole contributor to fixation suppression is not universally accepted. Some have argued that a separate and distinct mechanism, called vestibular cancellation, is responsible for suppression of vestibular nystagmus (e.g., Tomlinson and Robinson, 1981). They acknowledge that the pursuit and vestibular cancellation share many common neural pathways and both may contribute to fixation suppression. That is, the vestibular cancellation system is primarily responsible for reducing the nystagmus intensity and placing the target near the fovea. Subsequently, the pursuit system eliminates the residual eye movements. The supporters of this view point to the ability of afoveate animals to suppress vestibular nystagmus as one of the reasons that the two systems are not identical.
If we accept the latter view, the fixation suppression test is not redundant. In fact, a number of investigators have demonstrated that in some patients, the results of the fixation suppression test do not match the results from the tracking and the optokinetic tests (Barin and Davis, 2003). These cases are rare and do not prove that fixation suppression is mediated by a separate neural mechanism in addition to the smooth pursuit system. It may simply reflect the difference in the procedures for the tracking and fixation suppression tests.
Figure 2 shows a patient with clearly abnormal tracking for leftward target movements. Optokinetic results are consistent with the tracking test. The patient can track the target for rightward movements at 40 degrees per second, but fails to generate adequate nystagmus intensity even for the leftward stimulus at 20 degrees per second. However, this patient has normal fixation suppression for right-beating (leftward slow-phase) nystagmus. This case seems to support the notion of a distinct vestibular cancellation mechanism. However, a careful inspection of the results shows that the patient's pre-fixation nystagmus intensity was only 10 degrees per second. The optokinetic responses for leftward target movements show the patient is capable of generating nystagmus intensities of about 10 degrees per second. Therefore, this may simply reflect the fact that the fixation test is performed for the velocity range where the pursuit system is still intact. Similarly, there are alternative explanations for cases where fixation suppression is abnormal but the tracking test is normal. For example, sinusoidal and ramp target movements used in tracking and optokinetic tests are periodic and, therefore, have predictable trajectories. The stimulus to the pursuit system during fixation suppression is different because of the seemingly unpredictable timing of the caloric nystagmus fast phases. The pursuit performance is greatly enhanced for predictable stimuli, which may explain how a patient with normal tracking for predictable stimuli can have failure of fixation suppression.
The above discussion suggests that suppression of vestibular nystagmus is closely associated with the smooth pursuit system. However, in rare cases, there are patients who have normal smooth pursuit but fail to adequately suppress vestibular nystagmus and vice versa. Therefore, the fixation suppression test does not seem to be redundant because one can not rule out the possibility that a distinct vestibular cancellation mechanism is involved in fixation suppression.
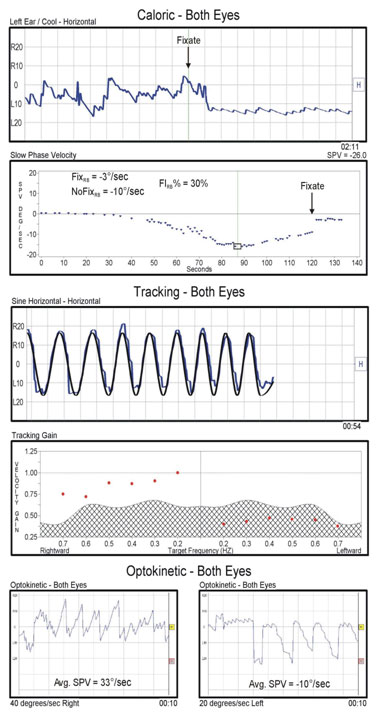
Figure 2. An example of a patient with normal fixation suppression and abnormal tracking and optokinetic tests.
Limitations
Testing fixation suppression during the caloric test has a number of limitations. First, based on the normative values for the tracking and optokinetic tests, the optimal pre-fixation nystagmus intensities for the fixation suppression test are between 20 degrees per second to 40 degrees per second. However, since the caloric stimulus is uncalibrated, it is not possible to control the strength of the response. If nystagmus intensities are much lower than 20 degrees per second, even patients with defective smooth pursuit or vestibular cancellation can suppress it. Nystagmus intensities that are much greater than 40 degrees per second can overwhelm even a normal fixation suppression system.
Second, the ability to suppress vestibular nystagmus is affected by the patient's age and gender (Jacobson, et al., 1993). However, these factors are not reflected in the fixation index. It is likely that the optimal pre-fixation nystagmus intensities are around 40 degrees per second for young individuals. However, normal values for the elderly may be closer to 20 degrees per second and that for higher nystagmus intensities, the fixation suppression test may be falsely identified as abnormal.
Finally, tracking and fixation are under voluntary control. Therefore, failure of fixation suppression may reflect the fact that the patient is unwilling to fixate and is not following test instructions. The examiner can recognize this more readily in the tracking and optokinetic tests but not in the fixation suppression test.
There is an alternative to testing fixation suppression during the caloric test for laboratories that have access to a rotary chair. The patient is rotated in both complete darkness and in the presence of a small LED light affixed to the chair. In the latter test, the patient is instructed to fixate on the LED light. The peak nystagmus intensities with fixation are compared to the peak nystagmus intensities in complete darkness to determine the fixation index. Since in rotary chair testing, the stimulus is precise and highly repeatable, pre-fixation nystagmus intensities can be controlled and normative values can be adjusted for age and gender.
Summary
The fixation suppression test is a test of central pathways, most likely those associated with the smooth pursuit system. The test has a number of limitations, which affect its usefulness and require more careful interpretation to avoid false positive and false negative results. Nonetheless, the fixation suppression test is a simple test to administer and does not require any additional test time. The test can sometimes detect abnormalities that are not identified in the tracking and optokinetic test and vice versa.
References
Alpert, J.N. (1974). Failure of fixation suppression: A pathologic effect of vision on caloric nystagmus. Neurology, 24, 891-896.
American National Standards Institute. (1999). Procedures for testing basic vestibular function. ANSI BSR S3.45-200X.
Baloh, R.W., & Honrubia, V. (1990). Clinical Neurophysiology of the Vestibular System. Philadelphia: FA Davis.
Barin, K. & Davis, L.R. (2003). The fixation suppression test. Insights in Practice. ICS Medical, Feb. 2003, pp. 1-5.
Barnes, G.R., Benson, A.J., & Prior, A.R.J. (1978). Visual-vestibular interaction in the control of eye movement. Aviation, Space, and Environmental Medicine, 49, 557-564.
Demanez, J.P., & Ledoux, A. (1970). Automatic fixation mechanisms and vestibular stimulation. Their study in central pathology with ocular fixation index during caloric tests. Advances in Oto-Rhino-Laryngology, 17, 90-8.
Halmagyi, G.M., and Gresty, M.A. (1979). Clinical signs of visual-vestibular interaction. Journal of neurology, neurosurgery, and psychiatry, 42(10), 934-939.
Jacobson, G.P., Newman, C.W., & Peterson, E.L. (1993). Interpretation and usefulness of caloric testing. In Jacobson, G.P., Newman, C.W., & Kartush, J.M. (Eds.), Handbook of Balance Testing Function (pp. 193-233). St. Louis: Mosby Year Book.
Tomlinson, R.D., & Robinson, D.A. (1981). Is the vestibulo-ocular reflex cancelled by smooth pursuit? In Fuchs, A. and Becker W. (Eds.), Progress in Oculomotor Research (pp. 533-539). New York: Elsevier/North-Holland.


