Interview with Michael Raffin Ph.D., Director of Audiology, Associate Professor, Otolaryngology, Loyola University Medical Center
Topic: Intraoperative ABR ...and related issues
Beck: Hi Michael, thanks for joining me today.
Raffin: Hi Doug, thanks for inviting me.
Beck: Michael, would you please review your education and your early career for me?
Raffin: Sure, no problem at all. I earned my undergraduate degree in French and then I was drafted into the military and did a tour in Viet Nam. After I changed from machine-gunner to medic, I wound up getting wounded. When I got out of the military I was interested in being a physician's assistant and I was working with an eye-ear-nose and throat doc, and pretty soon I became totally interested in ears, and went to graduate school for audiology in Los Angeles, and went to Iowa for my Ph.D., which is where I met Aaron Thornton. After I earned my doctorate in 1976, I worked at several places and eventually went to Boston and worked with Dr. Thornton at Massachusetts Eye and Ear.
Beck: When did you start at Loyola?
Raffin: 1993. In fact they were just re-structuring the department and they had ended the Speech and Hearing Clinic model, and when I started, I became the Director of Audiology, and it's worked out quite well for all of us. We do everything clinically from hearing aids and implants to ABR and OAE. We have ten audiologists and we staff three satellite site locations, and as you know, I also monitor one or two intraoperative cases per week too.
Beck: What are the primary types of surgical cases you monitor?
Raffin: Probably some 90 plus percent of the cases I'm involved with are cerebellopontine angle tumors such as acoustic neuromas and meningiomas. Then again, we do another one or two cases in the OR for threshold determination every month or so.
Beck: It might be good to mention that for the majority of acoustic neuromas, the surgical approach is translabyrinthine, which means drilling through the inner ear to access and remove the tumor. So the bottom line is, there's no point monitoring those cases as we know the hearing will be sacrificed to safely remove the tumor. The cases you monitor are "hearing preservation" cases, which will generally limit you to tumors approached via retrosigmoid and middle fossa approaches.
Raffin: Yes, very good point. And of our hearing preservation cases, probably some 2/3rds are retrosigmoid and the rest are middle fossa. We also had a few modified translab. Approaches, so those are interesting too.
Beck: And of the tumors you do monitor, how do you decide which ones to attempt to monitor and which ones aren't going to be monitored?
Raffin: Those decisions are made preoperatively. Basically, candidacy for hearing preservation implies the tumor is small, and they obviously have hearing to begin with, if we're going to try to preserve it! So the decision as to whether or not we monitor hearing in the OR has to do with whether or not we can get an ABR in the office. If the ABR appears robust in the office, we'll try to monitor it during surgery. If we cannot get an ABR in the office, we don't monitor that case in the OR.
Beck: What type of recording electrodes do you use in the OR?
Raffin: I use the standard silver-chloride cup electrodes, using the vertex positive, just at the hairline. The negative electrode is placed in front of the tragus, to stay out of the way of the surgical procedure itself, and the ground is usually on the shoulder. We tape everything down and we have to be very careful to make sure the wires are out of the way, safe and secure. Of course, sometimes, depending on the case and the situation, we have to move things around.
Beck: When you're doing intraoperative monitoring, what are the features you look for in your monitoring equipment? What are the options and features that appeal to you?
Raffin: With respect to features, I am highly focused on optimizing the signal-to-noise ratio during recording. With respect to the electrode, we make our own shielded electrode from commercially available stock electrodes and co-axial cable. Recently, Van Rijn and associates at Biosemi.com developed an active electrode whereby the preamp is "epoxyed" onto the electrode disc, and I think this has great promise. Unfortunately, the associated instrumentation does not come with AEP software. While we use a single channel montage, we actually use two channel recordings. The inputs of the second channel are "jumped" at the electrode box to be 180 degrees out of phase with that of Channel One, and therefore, Channel Two's positive input is Channel One's negative input. I would like a pre-amp built into the electrode box or connector assembly, but this is not the standard across manufacturers. By the time our recorded signals get to the A/D converter, Channel One's electrode signals are out-of-phase with Channel Two's electrode signals, but noise gathered between the electrode box and the A/D converter is in phase. So, then with software, we invert Channel Two, which brings Channel Two's noise out-of-phase, relative to Channel One's noise, while returning the signal to in-phase across the two channels, and then we add the two channels together. We'll attach some illustrations which make it more clear. Also important, is the ability to keep separated responses to rarefaction stimuli from responses to condensation stimuli, be it from the software mode of operation or through an adaptation of the P-300 paradigm. In the OR, this is important because sometimes responses to one stimulus polarity are detectable sooner than responses to the other polarity. The stimulus polarity which generates the fastest or cleanest responses is highly variable from patient to patient and is not predictable. Also important is to have some metric of signal-to-noise ratio, command over display gain; pausing and either restarting or continuing any given average instantaneously as well as ability to save a given trace without having to start a new average. It would be nice to eliminate the hardwire connections from the electrodes and earphones to the analysis device by using wireless technology, but I have not found such an instrument. Finally, the less a given manufacturer imposes on me, the happier I am ... So I prefer to have complete control over every single detail of the instrument setup, even though I may never exercise some of those options or change them from default settings.
Beck: And what do you use to deliver the clicks to the ear?
Raffin: Standard insert earphones with a standard plastic tube, that's long enough for our purposes.
Beck: When do you record the ABR?
Raffin: We do the first baseline recording as soon as we have everything hooked up and before the patient is draped. After that, we perform the ABR strategically when the surgeon is getting near the nerve or the related structures. Another issue is that we only need to do a few hundred sweeps in the OR at 33.3 clicks per second, so it only takes 3 to 8 seconds to obtain the data. Of course in the clinic you may need to do thousands of clicks, but in the OR the patient is very quiet physiologically, and so a smaller sample is all that is required.
Beck: Exactly, there are three goals to anesthesia; no memory, no motion and no pain. So the patient is indeed very quiet while intubated and anesthetized! But then again, to make up for the quiet patient, you have the electrically noisy OR!
Raffin: Yes, that's true. Most of the equipment in the OR is very noisy electrically and that can be a real issue. Even the surgical microscope gives off a tremendous 60 cycle noise, over 200 milliTesla of magnetic flux density at the ear, according to our probe measurements, and that makes it very difficult to obtain an intraoperative ABR. Another noise source is the electric blanket the nurses and the anesthesiologists place around the patient. We usually have to turn the blankets off or we cannot record the ABR. Other noise sources include the new wireless systems and the proximity of electrode wires to metal objects and this is most noticeable with unshielded electrode wires.
Beck: When do you notify the surgeon that a change in the ABR has occurred?
Raffin: That's an excellent question. Sometimes we use a criterion of a 50 percent change in amplitude, sometimes even a slight shift or larger shift in latency, and sometimes, it just depends on the situation at hand. In other words, the best calls we make are based on the context, knowing the anatomy, and knowing what the surgeon is doing, and incorporating our "early warning" protocol into the context.
Beck: That's a great point for many reasons. I can recall many cases when the surgeon was a million miles away from the ear, and the ABR would change for any of a thousand reasons, none of which had anything to do with real damage to the ear or hearing! For example, the patient's body temperature might change, irrigation fluid might migrate under the earphone or into the middle ear, you might have negative pressure in the middle ear space attributable to anesthesia, the electrodes might be wiggled accidentally, the electro-cautery equipment may obliterate the response temporarily....any of those can impact the ABR, and there are dozens of other things that impact the ABR too. But if I were to say "the ABR changed" while they were still creating the surgical exposure - I'd look pretty silly!
Raffin: Exactly right Doug. And that's another reason we need to really know the anatomy. Looking at an ABR response out of context has little meaning.
Beck: Kind of like reading a map without a scale of miles.
Raffin: Right. A two-inch squiggly line might be two miles or 2000 miles.
Beck: What about the ABR while the surgeon is drilling away the internal auditory canal?
Raffin: Well, as you know, the ABR can go all over the place. The noise from the drill can mess up the ABR, as can the heat generated from the drill and even the irrigation fluid and its temperature can cause the ABR to go bonkers. Then again, as soon as I see a change in the ABR, if I know the surgeon is working on or near the auditory nerve, I keep them posted as to my observations and they have to decide which information is the most important to them, and what they might do about it. In other words, they have to use the information I give them and decide what to do about the changes in the ABR -- if anything at all. The surgeon has to make the decision based on the entire event, the surgery, the patient's safety and the confidence that he or she may have in the ABR, or the person reporting the ABR! ABR has false negatives and false positives, and the surgeon has to make the decision.
Beck: One thing I always point out to my students is that the ABR can actually go to a flat line -- and interestingly, that does not always mean the hearing has been compromised. In fact, in my book from ten years ago, the "Handbook of Intraoperative Monitoring," we offered a case study of an ABR during a vestibular nerve section, and the ABR went away totally while the surgeon was sectioning the nerve, and it came back a little while later, while suturing up the dura and the post-op outcome was normal hearing. Sometimes, it's the issues you raised above, irrigation fluid, heat, noise from the drill etc, and sometimes it's neural dysynchrony, from tugging on the auditory fibers while sectioning the vestibular fibers -- and that does keep it very challenging. But I think you nailed it, it's all about context and knowing what is going on during the surgical procedure and how that relates to the ABR, or really any modality being monitored.
Raffin: I agree totally. Anatomic knowledge is key. One cannot be in the OR as a "tech" just knowing how to do the test, without understanding the anatomy and the physiology. It all matters.
Beck: And speaking of things that matter...does ABR in the operating room matter? Is it really worth going through all of this?
Raffin: Yes. it really is. ABR does correlate with post-op hearing. About 86 percent of the time, when I think we've preserved hearing, we have done so with no changes in the hearing . Now there is a difference in preserving hearing and conserving hearing! If our ABR says the hearing is gone, we'll be right 95 percent of the time. Our overall efficiency of correctly predicting hearing conservation is about 87.5%. So yes, the intraoperative ABR does matter, if done well and taken in context.
Beck: Michael, it's been a pleasure speaking with you. I hope we're able to follow-up soon.
Raffin: Thanks Doug. It's a pleasure speaking with you too.
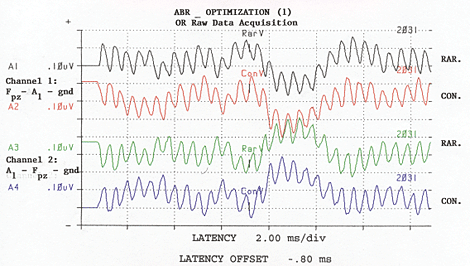
Figure 1: 2,031 sweeps, Channels One signals are 180 degrees with those of channel Two, and acquired noise from electrode box is in phase. Adapted from: Raffin, M.J.M., Hansen, J.M. & Sinclair, J.S., (1992): Enhanced EcochG and Méniére's Disease: Clinical Fantasy in Dehydration. Paper presented at the Annual Convention of the American Speech-Language-Hearing Association, San Antonio, TX, 23 November 1992.
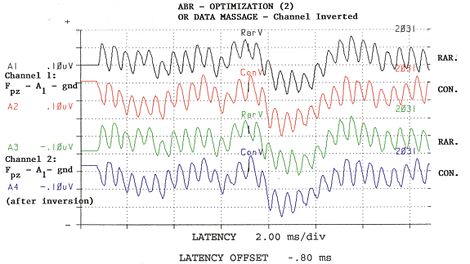
Figure 2:
Software inversion of Channel Two: Channel One and Channel Two signals are now in phase and acquired noise from the electrode box is out-of-phase between the two channels. Adapted from: Raffin, M.J.M., Hansen, J.M. & Sinclair, J.S., (1992): Enhanced EcochG and Méniére's Disease: Clinical Fantasy in Dehydration. Paper presented at the Annual Convention of the American Speech-Language-Hearing Association, San Antonio, TX, 23 November 1992.
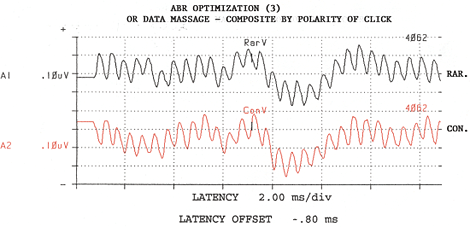
Figure 3: Responses to rarefaction stimuli from Channel One are added to responses from rarefaction stimuli from Channel two. Likewise for responses to Condensation stimuli. Composite number of sweeps is now 4,062. Adapted from: Raffin, M.J.M., Hansen, J.M. & Sinclair, J.S., (1992): Enhanced EcochG and Méniére's Disease: Clinical Fantasy in Dehydration. Paper presented at the Annual Convention of the American Speech-Language-Hearing Association, San Antonio, TX, 23 November 1992.
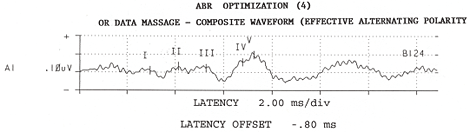
Figure 4: Composite rarefaction responses are added to composite condensation responses to yield the composite alternating-stimulus response from 8,124 sweeps. Adapted from: Raffin, M.J.M., Hansen, J.M. & Sinclair, J.S., (1992): Enhanced EcochG and Méniére's Disease: Clinical Fantasy in Dehydration. Paper presented at the Annual Convention of the American Speech-Language-Hearing Association, San Antonio, TX, 23 November 1992.
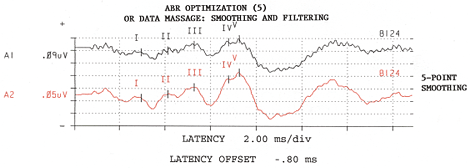
Figure 5: Composite alternating-polarity response is smoothed. Adapted from: Raffin, M.J.M., Hansen, J.M. & Sinclair, J.S., (1992): Enhanced EcochG and Méniére's Disease: Clinical Fantasy in Dehydration. Paper presented at the Annual Convention of the American Speech-Language-Hearing Association, San Antonio, TX, 23 November 1992.

Figure 6: Display aspect ratio of the smoothed composite changed to enhance visual recognition (or illusions due entirely to personal predispositions) - the data are identical, but eye-candy effects may be significant. Adapted from: Raffin, M.J.M., Hansen, J.M. & Sinclair, J.S., (1992): Enhanced EcochG and Méniére's Disease: Clinical Fantasy in Dehydration. Paper presented at the Annual Convention of the American Speech-Language-Hearing Association, San Antonio, TX, 23 November 1992.
Raffin: Hi Doug, thanks for inviting me.
Beck: Michael, would you please review your education and your early career for me?
Raffin: Sure, no problem at all. I earned my undergraduate degree in French and then I was drafted into the military and did a tour in Viet Nam. After I changed from machine-gunner to medic, I wound up getting wounded. When I got out of the military I was interested in being a physician's assistant and I was working with an eye-ear-nose and throat doc, and pretty soon I became totally interested in ears, and went to graduate school for audiology in Los Angeles, and went to Iowa for my Ph.D., which is where I met Aaron Thornton. After I earned my doctorate in 1976, I worked at several places and eventually went to Boston and worked with Dr. Thornton at Massachusetts Eye and Ear.
Beck: When did you start at Loyola?
Raffin: 1993. In fact they were just re-structuring the department and they had ended the Speech and Hearing Clinic model, and when I started, I became the Director of Audiology, and it's worked out quite well for all of us. We do everything clinically from hearing aids and implants to ABR and OAE. We have ten audiologists and we staff three satellite site locations, and as you know, I also monitor one or two intraoperative cases per week too.
Beck: What are the primary types of surgical cases you monitor?
Raffin: Probably some 90 plus percent of the cases I'm involved with are cerebellopontine angle tumors such as acoustic neuromas and meningiomas. Then again, we do another one or two cases in the OR for threshold determination every month or so.
Beck: It might be good to mention that for the majority of acoustic neuromas, the surgical approach is translabyrinthine, which means drilling through the inner ear to access and remove the tumor. So the bottom line is, there's no point monitoring those cases as we know the hearing will be sacrificed to safely remove the tumor. The cases you monitor are "hearing preservation" cases, which will generally limit you to tumors approached via retrosigmoid and middle fossa approaches.
Raffin: Yes, very good point. And of our hearing preservation cases, probably some 2/3rds are retrosigmoid and the rest are middle fossa. We also had a few modified translab. Approaches, so those are interesting too.
Beck: And of the tumors you do monitor, how do you decide which ones to attempt to monitor and which ones aren't going to be monitored?
Raffin: Those decisions are made preoperatively. Basically, candidacy for hearing preservation implies the tumor is small, and they obviously have hearing to begin with, if we're going to try to preserve it! So the decision as to whether or not we monitor hearing in the OR has to do with whether or not we can get an ABR in the office. If the ABR appears robust in the office, we'll try to monitor it during surgery. If we cannot get an ABR in the office, we don't monitor that case in the OR.
Beck: What type of recording electrodes do you use in the OR?
Raffin: I use the standard silver-chloride cup electrodes, using the vertex positive, just at the hairline. The negative electrode is placed in front of the tragus, to stay out of the way of the surgical procedure itself, and the ground is usually on the shoulder. We tape everything down and we have to be very careful to make sure the wires are out of the way, safe and secure. Of course, sometimes, depending on the case and the situation, we have to move things around.
Beck: When you're doing intraoperative monitoring, what are the features you look for in your monitoring equipment? What are the options and features that appeal to you?
Raffin: With respect to features, I am highly focused on optimizing the signal-to-noise ratio during recording. With respect to the electrode, we make our own shielded electrode from commercially available stock electrodes and co-axial cable. Recently, Van Rijn and associates at Biosemi.com developed an active electrode whereby the preamp is "epoxyed" onto the electrode disc, and I think this has great promise. Unfortunately, the associated instrumentation does not come with AEP software. While we use a single channel montage, we actually use two channel recordings. The inputs of the second channel are "jumped" at the electrode box to be 180 degrees out of phase with that of Channel One, and therefore, Channel Two's positive input is Channel One's negative input. I would like a pre-amp built into the electrode box or connector assembly, but this is not the standard across manufacturers. By the time our recorded signals get to the A/D converter, Channel One's electrode signals are out-of-phase with Channel Two's electrode signals, but noise gathered between the electrode box and the A/D converter is in phase. So, then with software, we invert Channel Two, which brings Channel Two's noise out-of-phase, relative to Channel One's noise, while returning the signal to in-phase across the two channels, and then we add the two channels together. We'll attach some illustrations which make it more clear. Also important, is the ability to keep separated responses to rarefaction stimuli from responses to condensation stimuli, be it from the software mode of operation or through an adaptation of the P-300 paradigm. In the OR, this is important because sometimes responses to one stimulus polarity are detectable sooner than responses to the other polarity. The stimulus polarity which generates the fastest or cleanest responses is highly variable from patient to patient and is not predictable. Also important is to have some metric of signal-to-noise ratio, command over display gain; pausing and either restarting or continuing any given average instantaneously as well as ability to save a given trace without having to start a new average. It would be nice to eliminate the hardwire connections from the electrodes and earphones to the analysis device by using wireless technology, but I have not found such an instrument. Finally, the less a given manufacturer imposes on me, the happier I am ... So I prefer to have complete control over every single detail of the instrument setup, even though I may never exercise some of those options or change them from default settings.
Beck: And what do you use to deliver the clicks to the ear?
Raffin: Standard insert earphones with a standard plastic tube, that's long enough for our purposes.
Beck: When do you record the ABR?
Raffin: We do the first baseline recording as soon as we have everything hooked up and before the patient is draped. After that, we perform the ABR strategically when the surgeon is getting near the nerve or the related structures. Another issue is that we only need to do a few hundred sweeps in the OR at 33.3 clicks per second, so it only takes 3 to 8 seconds to obtain the data. Of course in the clinic you may need to do thousands of clicks, but in the OR the patient is very quiet physiologically, and so a smaller sample is all that is required.
Beck: Exactly, there are three goals to anesthesia; no memory, no motion and no pain. So the patient is indeed very quiet while intubated and anesthetized! But then again, to make up for the quiet patient, you have the electrically noisy OR!
Raffin: Yes, that's true. Most of the equipment in the OR is very noisy electrically and that can be a real issue. Even the surgical microscope gives off a tremendous 60 cycle noise, over 200 milliTesla of magnetic flux density at the ear, according to our probe measurements, and that makes it very difficult to obtain an intraoperative ABR. Another noise source is the electric blanket the nurses and the anesthesiologists place around the patient. We usually have to turn the blankets off or we cannot record the ABR. Other noise sources include the new wireless systems and the proximity of electrode wires to metal objects and this is most noticeable with unshielded electrode wires.
Beck: When do you notify the surgeon that a change in the ABR has occurred?
Raffin: That's an excellent question. Sometimes we use a criterion of a 50 percent change in amplitude, sometimes even a slight shift or larger shift in latency, and sometimes, it just depends on the situation at hand. In other words, the best calls we make are based on the context, knowing the anatomy, and knowing what the surgeon is doing, and incorporating our "early warning" protocol into the context.
Beck: That's a great point for many reasons. I can recall many cases when the surgeon was a million miles away from the ear, and the ABR would change for any of a thousand reasons, none of which had anything to do with real damage to the ear or hearing! For example, the patient's body temperature might change, irrigation fluid might migrate under the earphone or into the middle ear, you might have negative pressure in the middle ear space attributable to anesthesia, the electrodes might be wiggled accidentally, the electro-cautery equipment may obliterate the response temporarily....any of those can impact the ABR, and there are dozens of other things that impact the ABR too. But if I were to say "the ABR changed" while they were still creating the surgical exposure - I'd look pretty silly!
Raffin: Exactly right Doug. And that's another reason we need to really know the anatomy. Looking at an ABR response out of context has little meaning.
Beck: Kind of like reading a map without a scale of miles.
Raffin: Right. A two-inch squiggly line might be two miles or 2000 miles.
Beck: What about the ABR while the surgeon is drilling away the internal auditory canal?
Raffin: Well, as you know, the ABR can go all over the place. The noise from the drill can mess up the ABR, as can the heat generated from the drill and even the irrigation fluid and its temperature can cause the ABR to go bonkers. Then again, as soon as I see a change in the ABR, if I know the surgeon is working on or near the auditory nerve, I keep them posted as to my observations and they have to decide which information is the most important to them, and what they might do about it. In other words, they have to use the information I give them and decide what to do about the changes in the ABR -- if anything at all. The surgeon has to make the decision based on the entire event, the surgery, the patient's safety and the confidence that he or she may have in the ABR, or the person reporting the ABR! ABR has false negatives and false positives, and the surgeon has to make the decision.
Beck: One thing I always point out to my students is that the ABR can actually go to a flat line -- and interestingly, that does not always mean the hearing has been compromised. In fact, in my book from ten years ago, the "Handbook of Intraoperative Monitoring," we offered a case study of an ABR during a vestibular nerve section, and the ABR went away totally while the surgeon was sectioning the nerve, and it came back a little while later, while suturing up the dura and the post-op outcome was normal hearing. Sometimes, it's the issues you raised above, irrigation fluid, heat, noise from the drill etc, and sometimes it's neural dysynchrony, from tugging on the auditory fibers while sectioning the vestibular fibers -- and that does keep it very challenging. But I think you nailed it, it's all about context and knowing what is going on during the surgical procedure and how that relates to the ABR, or really any modality being monitored.
Raffin: I agree totally. Anatomic knowledge is key. One cannot be in the OR as a "tech" just knowing how to do the test, without understanding the anatomy and the physiology. It all matters.
Beck: And speaking of things that matter...does ABR in the operating room matter? Is it really worth going through all of this?
Raffin: Yes. it really is. ABR does correlate with post-op hearing. About 86 percent of the time, when I think we've preserved hearing, we have done so with no changes in the hearing . Now there is a difference in preserving hearing and conserving hearing! If our ABR says the hearing is gone, we'll be right 95 percent of the time. Our overall efficiency of correctly predicting hearing conservation is about 87.5%. So yes, the intraoperative ABR does matter, if done well and taken in context.
Beck: Michael, it's been a pleasure speaking with you. I hope we're able to follow-up soon.
Raffin: Thanks Doug. It's a pleasure speaking with you too.

Figure 1: 2,031 sweeps, Channels One signals are 180 degrees with those of channel Two, and acquired noise from electrode box is in phase. Adapted from: Raffin, M.J.M., Hansen, J.M. & Sinclair, J.S., (1992): Enhanced EcochG and Méniére's Disease: Clinical Fantasy in Dehydration. Paper presented at the Annual Convention of the American Speech-Language-Hearing Association, San Antonio, TX, 23 November 1992.

Figure 2:
Software inversion of Channel Two: Channel One and Channel Two signals are now in phase and acquired noise from the electrode box is out-of-phase between the two channels. Adapted from: Raffin, M.J.M., Hansen, J.M. & Sinclair, J.S., (1992): Enhanced EcochG and Méniére's Disease: Clinical Fantasy in Dehydration. Paper presented at the Annual Convention of the American Speech-Language-Hearing Association, San Antonio, TX, 23 November 1992.

Figure 3: Responses to rarefaction stimuli from Channel One are added to responses from rarefaction stimuli from Channel two. Likewise for responses to Condensation stimuli. Composite number of sweeps is now 4,062. Adapted from: Raffin, M.J.M., Hansen, J.M. & Sinclair, J.S., (1992): Enhanced EcochG and Méniére's Disease: Clinical Fantasy in Dehydration. Paper presented at the Annual Convention of the American Speech-Language-Hearing Association, San Antonio, TX, 23 November 1992.

Figure 4: Composite rarefaction responses are added to composite condensation responses to yield the composite alternating-stimulus response from 8,124 sweeps. Adapted from: Raffin, M.J.M., Hansen, J.M. & Sinclair, J.S., (1992): Enhanced EcochG and Méniére's Disease: Clinical Fantasy in Dehydration. Paper presented at the Annual Convention of the American Speech-Language-Hearing Association, San Antonio, TX, 23 November 1992.

Figure 5: Composite alternating-polarity response is smoothed. Adapted from: Raffin, M.J.M., Hansen, J.M. & Sinclair, J.S., (1992): Enhanced EcochG and Méniére's Disease: Clinical Fantasy in Dehydration. Paper presented at the Annual Convention of the American Speech-Language-Hearing Association, San Antonio, TX, 23 November 1992.

Figure 6: Display aspect ratio of the smoothed composite changed to enhance visual recognition (or illusions due entirely to personal predispositions) - the data are identical, but eye-candy effects may be significant. Adapted from: Raffin, M.J.M., Hansen, J.M. & Sinclair, J.S., (1992): Enhanced EcochG and Méniére's Disease: Clinical Fantasy in Dehydration. Paper presented at the Annual Convention of the American Speech-Language-Hearing Association, San Antonio, TX, 23 November 1992.

