A colleague once pointed out to me that the audiogram is one of the most powerful diagnostic tests in medicine (David Zapala, Ph.D., personal communication). It influences the diagnosis and selection of treatments in direct and profound ways. Choices of surgery, medical treatment, hearing aids, cochlear implants, and the need for other diagnostic procedures are based heavily on the audiogram. Audiologists are keenly aware of the importance of audiometric findings and take measures to ensure the accuracy of the results before sending them to other health professionals.
Perhaps the most important diagnostic feature of the audiogram is the air-bone gap. By distinguishing between conductive and sensorineural impairments, the air-bone gap profoundly influences the patient's care. A false air-bone gap can lead to inappropriate medical or surgical treatment. A missed air-bone gap can cause harmful delays in medical management. It is important that audiologists take great care in determining if air-bone gaps are accurate.
Audiologists are taught that normal hearing patients and those with sensorineural hearing loss do not exhibit air-bone gaps. On the average this is true;however, audiologists have to consider the variability of air and bone conduction thresholds. Because of this variability of threshold measurements, audiologists should not expect that all air-bone gaps in these patients will be zero. In fact, that should be the exception, not the rule.
Variability of Air-Bone Gaps
Consider the audiogram in Figure 1. Bone conduction thresholds were tested at four frequencies (.5, 1, 2 and 4 kHz). The air-bone gaps are zero at all four frequencies in both the right ear and left ear, which is a total of eight frequencies. In reality, the probability that the air-bone gaps are zero at four frequencies is about two in a thousand, and the probability that all eight air-bone gaps are zero is four in a million.
Studebaker (1967) pointed out four decades ago that if the average air-bone gap is a normally-distributed variable with a standard deviation of 5 dB then the value is zero only 38% of the time. The probability that four air-bone gaps would be zero is 0.384 = 0.02 (2%). Studebaker correctly warned that air-bone gaps of 10, 15 and 20 dB should be expected for a significant proportion of threshold measurements, and these should not be over-interpreted as evidence of middle ear dysfunction.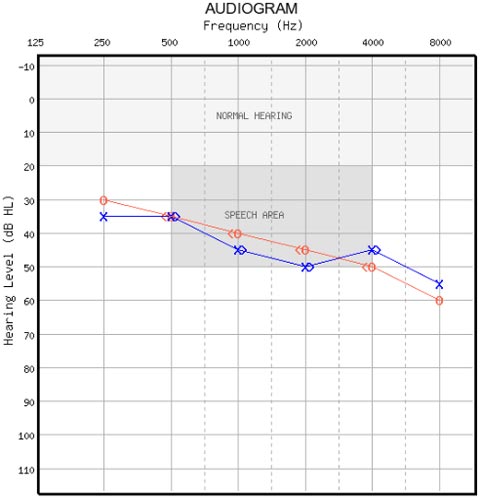
Figure 1. What is wrong with this audiogram?
It is perhaps more realistic to consider the air-bone gap as a difference between two normally-distributed variables - the air conduction threshold and the bone conduction threshold. After assessing duplicate audiograms obtained by two audiologists on the same patients, we determined that reasonable estimates of the standard deviation for air conduction and bone conduction thresholds are 5 dB and 7 dB, respectively. The resulting distribution of thresholds is shown in Figure 2.
If the sources of variability for air conduction and bone conduction are independent, thresholds can be considered a pair of values randomly selected, one from each of the distributions in Figure 2. The standard deviation of the distribution of differences is the square root of the sum of the two squared values: √;;;;;;(a2 + b2) = 8.6 dB, where a and b are the standard deviations for air and bone conduction. This is quite a bit larger than Studebaker's 5 dB assumption, and it leads to an expectation of larger and more frequent air-bone gaps in patients with normal middle-ear function. The distribution of air-bone gaps is shown in Figure 3. Almost a quarter of the time (23%) the difference between air and bone conduction thresholds is 10 dB in one direction or the other. Furthermore, 15 dB differences occur 12% of the time. Differences of 10 dB or more (both positive and negative) occur 40% of the time.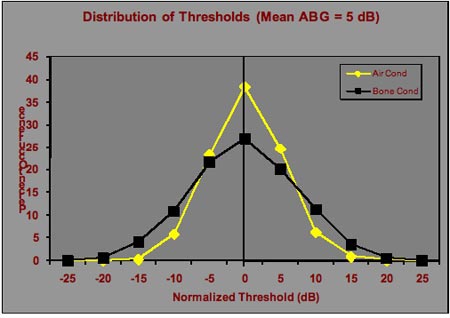
Figure 2. Distributions of air and bone conduction thresholds that would occur on repeated testing of an individual patient with normal hearing or sensorineural hearing loss with an air-bone gap of 0 dB.
Figures 2 and 3 assume an average air-bone gap of zero;however, it is perfectly plausible that a patient may have a large ear canal, less efficient coupling of the earphone to the head, a sluggish ossicular chain, or any number of other situations that could result in better hearing by bone than by air. Therefore, Figure 4 shows the distribution of air and bone conduction thresholds considering an average -5 dB air-bone gap, and Figure 5 shows the corresponding distribution of air-bone gaps. Air-bone gaps of 15 dB or more occur almost 20% of the time. Differences of 10 dB or more (both positive and negative) occur 48% of the time.
Based on these calculations, the probability of getting an audiogram like Figure 1 for one ear is 0.002 and for both ears is 0.000004.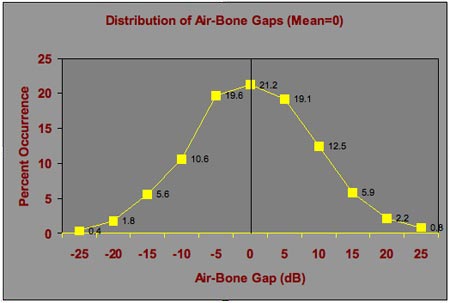
Figure 3. Distribution of air-bone gaps based on the air and bone conduction threshold distributions in Figure 2. The figure shows the variation in air-bone gaps that are expected on repeated testing of an individual patient when the average air-bone gap is zero.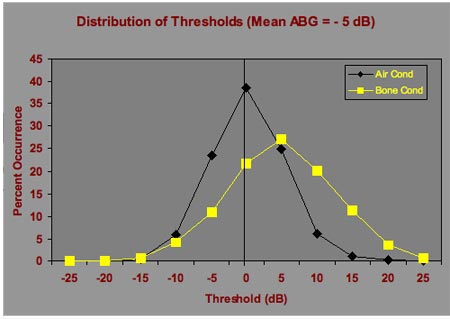
Figure 4. Distributions of air and bone conduction thresholds that would occur on repeated testing of an individual patient with normal hearing or sensorineural hearing loss with an air-bone gap of -5 dB.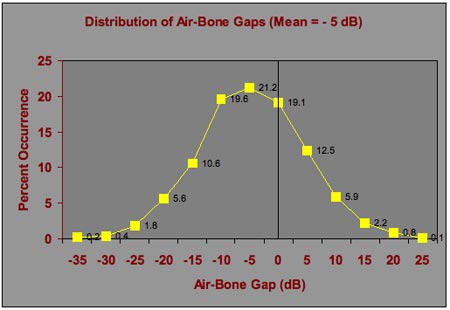
Figure 5. Distribution of air-bone gaps based on the air and bone conduction threshold distributions in Figure 4. The figure shows the variation in air-bone gaps that are expected on repeated testing of an individual patient when the average air-bone gap is -5 dB.
The Dirty Little Secret
What is the dirty little secret? The dirty little secret is that bone conduction testing is a biased experiment. Most of the time, the audiologist knows or has a strong suspicion of whether there should be an air-bone gap. This knowledge comes from previous audiograms, case history, thresholds that were just tested at other frequencies, and other results such as tympanometry and otoscopy. In any respectable research protocol, the investigator would never consider telling the tester the expected results. That would bias the outcome. Clinical trials, at great cost, are designed as double-blinded protocols in which neither the tester nor the subject knows the expected outcome. Expectations can influence the results even when the tester makes a concerted and genuine effort to be unbiased. When the true result is desired, the investigator and the subject must be "blind".
Given what was said in the first paragraphs about the importance of air-bone gaps, it is understandable that audiologists might bias the results toward what they know to be the correct result, thereby leading to the correct diagnosis and management of the patient. The result of this understandable and responsible approach is that audiologists and their "customers" (i.e., otolaryngologists), are accustomed to looking at biased data and are concerned when air-bone gaps are inconsistent with expectations. In reality, rather than questioning these natural air-bone gaps that should be expected during testing, the audiogram shown in Figure 1 should be the one questioned .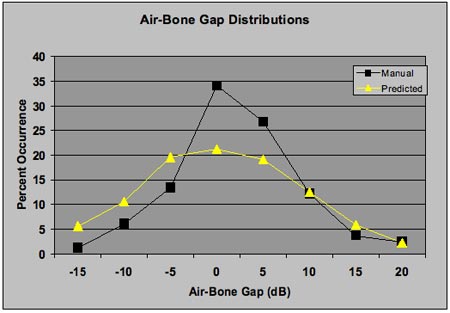
Figure 6. Distribution of air-bone gaps for a set of audiograms on patients with sensorineural hearing loss. Air-bone gaps from manual audiometry (squares) by an expert audiologist and the predicted distribution from Figure 3 (triangles) are shown. There are more air-bone gaps of 0 and 5 dB and fewer of -5, -10, and -15 in the manually-obtained audiograms compared to the predicted distribution.
Figure 6 shows evidence that this bias exists in audiometric testing. The figure shows the distribution of air-bone gaps (at 0.5, 1.0, and 2.0 kHz) from a group of 11 patients with sensorineural hearing loss who were tested by an expert audiologist. In addition, the figure displays the expected distribution from Figure 3. The 4-kHz air-bone gaps were omitted from this analysis for reasons discussed in the next section. As shown, there are more air-bone gaps of 0 and 5 dB and fewer of -5, -10, and -15 dB in the manually-obtained audiograms compared to the predicted distribution, which results in a narrower distribution of air-bone gaps in the manually-obtained audiograms. This is possibly a reflection of tester bias.
The 4 kHz Problem
For many years, U.S. and European literature has discussed an anomaly seen in bone conduction results (Frank & Holmes, 1981;Fagelson & Martin, 1994;Harkrider & Martin, 1998). Specifically, many clinicians have observed aberrant air-bone gaps at 4 kHz in patients that do not have air-bone gaps at other frequencies and have no other findings consistent with conductive hearing loss. Some clinics and calibration services intentionally deviate from the audiometer standard (American National Standards Institute, 2004) by reducing the 4 kHz bone conduction calibration level so that these aberrant air-bone gaps do not occur. Some have attributed this finding to acoustic radiation from the bone vibrator reaching the test ear by air conduction, causing an increase in the stimulus level reaching the cochlea and decreasing the bone conduction threshold. This explanation is supported by the data in Figure 7.
Figure 7 shows ear canal sound pressure measurements from one subject with a bone conduction stimulus delivered to the mastoid by the standard audiometric bone vibrator (Radioear B71) and the ear canal either open or occluded by a foam earplug. The higher sound pressure at low frequencies with the ear plugged is the well-known occlusion effect. Above 2 kHz, the sound pressure in the ear canal is substantially higher with the ear open than with the ear plugged, evidence of acoustic radiation of sound from the bone vibrator to the ear canal. The added sound pressure from acoustic radiation from the bone vibrator has the potential to artificially lower the bone conduction threshold at 4 kHz;however, this will only occur if the added energy from acoustic radiation significantly increases the level that reaches the cochlea. If the signal reaching the cochlea by bone conduction is equal to or greater than the sound that reaches the cochlea originating from acoustic radiation, no significant influence on threshold is expected. Moreover, multiple studies suggest that the acoustic radiation from the bone vibrator is not sufficiently intense to affect the bone conduction threshold at 4 kHz (Frank & Holmes, 1981;Harkrider & Martin, 1998).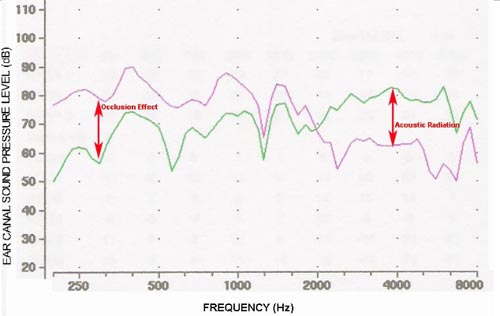
Figure 7. Ear canal sound pressure level measurements for a bone conducted stimulus delivered to the mastoid with the ear canal open (green) and plugged (pink). The low-frequency occlusion effect is an increase in sound pressure resulting from occluding the ear canal. The acoustic radiation effect is evident from the higher ear canal sound pressure when the ear is open compared to the plugged condition that blocks the sound reaching the ear canal by air conduction.
When the bone vibrator is placed on the forehead, the acoustic radiation to the ear canal is much less than when compared to mastoid placement;therefore, Figure 8 assesses ear canal sound pressure levels for the same subject produced by a bone vibrator on the forehead. Similarly to Figure 7, testing was completed with the ears open and when covered with circumaural earphones (Sennheiser HDA 200). The earphones are mounted in an enclosure that by itself is a hearing protection device that produces about 40 dB of attenuation of ambient sounds in the high frequencies. Because there is a large volume of air in the enclosure and it does not occlude the ear canal, the low-frequency occlusion effect is minimal. In the high frequencies, the acoustic radiation effect is much less than when compared to mastoid placement and is 0 dB at 2 and 4 kHz.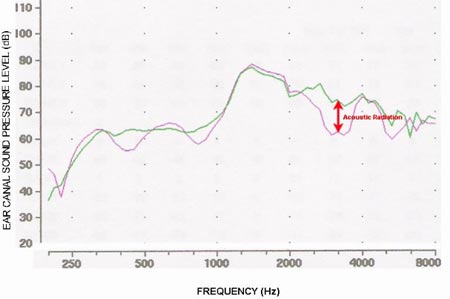
Figure 8. Ear canal sound pressure level measurements for a bone conducted stimulus delivered to the forehead with the ear canal open (green) and covered with a circumaural earphone (pink). The low frequency occlusion effect is minimal (compare to Figure 7). The acoustic radiation effect is much smaller when the bone vibrator is on the forehead.
If the aberrant 4-kHz air-bone gap results from acoustic radiation, then it should be much less when the bone vibrator is on the forehead, which is not the case. The same aberrant air-bone gaps occur with both forehead and mastoid placement. Figure 9 shows the average air-bone gaps from 11 patients with sensorineural hearing loss tested manually by an expert audiologist with mastoid placement of the bone vibrator and by an unbiased automated procedure with forehead placement. During the forehead bone measurements, the possibility of acoustic radiation from the bone vibrator to the ear canal was completely eliminated because a) the vibrator was on the forehead and b) both ears were covered by circumaural earphones. The earphone over the test ear blocked the acoustic radiation from the bone vibrator and the earphone over the non-test ear delivered masking. The average air-bone gaps are within 4 dB for the two procedures at 0.5, 1, and 2 kHz but differ by almost 10 dB at 4 kHz. The air-bone gap was not diminished with forehead bone testing relative to mastoid bone testing. In fact, Figure 9 shows larger air-bone gaps with the unbiased forehead procedure compared to a potentially-biased mastoid procedure. These data do not support the acoustic radiation explanation of aberrant air-bone gaps at 4 kHz, because they were even larger when there was no opportunity for radiation from the bone vibrator to affect bone conduction thresholds. The smaller air-bone gaps obtained with mastoid placement are evidence of tester bias (the dirty little secret).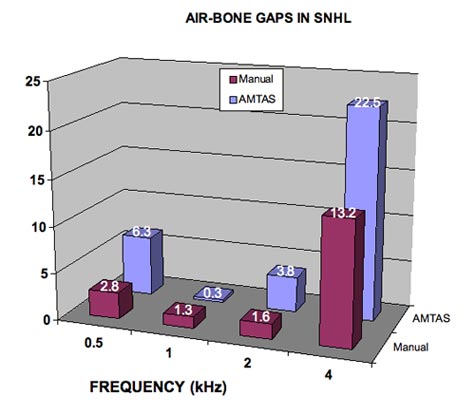
Figure 9. Average air-bone gaps for 11 patients with sensorineural hearing loss with manual audiometry and an unbiased automated method (AMTAS). The aberrant air-bone gaps at 4 kHz are larger with the automated procedure.
Comparisons of 4 kHz bone conduction thresholds with the test ear plugged and unplugged provide evidence that acoustic radiation is not the culprit. Frank and Holmes (1981) measured mastoid bone thresholds at 4 kHz with the test ear plugged and unplugged. If the threshold is artificially lowered by acoustic radiation from the vibrator, plugging the ear canal would elevate the threshold. They reported an average difference of less than 1 dB in the plugged and unplugged conditions, indicating that acoustic radiation from the vibrator placed on the mastoid does not contribute to bone conduction hearing at 4 kHz. Stenfelt and Reinfeldt (2007) measured ear canal sound pressure and bone conduction thresholds with the test ear plugged and unplugged and found that plugging the ear reduced the ear canal sound pressure above 2 kHz (evidence of acoustic radiation), but there was no change in the bone conduction threshold (evidence that the acoustic radiation does not affect threshold).
Conclusion
During testing, audiologists need to be aware of their own possible bias to meet false expectations of a 0 dB air-bone gap for normal hearing patients and patients with sensorineural hearing loss. The probability of a 0 dB air-bone gap at all frequencies is very small, and the true expectation should be to have some difference between air and bone conduction thresholds. Furthermore, depending on the frequency, this difference might be larger than expected, as can be seen with 4 kHz. In addition to other factors, the evidence suggests that there is acoustic radiation from a bone vibrator to the ear canal that is dependent on the location of the vibrator and the stimulus frequency;however, it does not affect bone conduction thresholds. The source of the aberrant 4 kHz bone conduction threshold is probably not acoustic radiation from the bone vibrator. We hope to shed some light on the real source in a future publication.
References
American National Standards Insitute (2004). American National Standard Specification for Audiometers. ANSI S3.6-2004, Acoustical Society of America: Melville, New York.
Frank, T. & Holmes, A. (1981). Acoustic radiation from bone vibrators. Ear and Hearing 2, 59-63.
Fagelson, M. & Martin, F.N. (1994). Sound pressure in the external auditory canal during bone-conduction testing. Amer. Acad. Audiol. 5, 379-383.
Harkrider, A.W. & Martin, F.N. (1998). Quantifying air-conducted acoustic radiation from the bone-conduction vibrator. J. Amer. Acad. Audiol. 9, 410-416.
Stenfelt, S.& Reinfeldt, S. (2007). A model of the occlusion effect with bone-conducted stimulation. International J Audiology 46, 595-608.
Studebaker, G.A. (1967). Intertest variability and the air-bone gap. J. Speech Hear. Dis. 32, 82-86.
The Vanishing Air-Bone Gap - Audiology's Dirty Little Secret
October 20, 2008
Related Courses
1
https://www.audiologyonline.com/audiology-ceus/course/masking-pure-and-simple-24973
Masking: Pure and Simple
This presentation will cover the importance of and scientific foundation behind clinical masking. The presenter will emphasize rules for when to mask, as well as common procedures used, with the provision of case scenarios throughout.
auditory, textual, visual
129
USD
Subscription
Unlimited COURSE Access for $129/year
OnlineOnly
AudiologyOnline
www.audiologyonline.com
Masking: Pure and Simple
This presentation will cover the importance of and scientific foundation behind clinical masking. The presenter will emphasize rules for when to mask, as well as common procedures used, with the provision of case scenarios throughout.
24973
Online
PT60M
Masking: Pure and Simple
Presented by L. Maureen Valente, PhD
Course: #24973Level: Intermediate1 Hour
AAA/0.1 Intermediate; ACAud inc HAASA/1.0; AHIP/1.0; BAA/1.0; CAA/1.0; Calif. SLPAB/1.0; IHS/1.0; Kansas, LTS-S0035/1.0; NZAS/1.0; SAC/1.0; TX TDLR, #142/1.0 Non-manufacturer
This presentation will cover the importance of and scientific foundation behind clinical masking. The presenter will emphasize rules for when to mask, as well as common procedures used, with the provision of case scenarios throughout.
2
https://www.audiologyonline.com/audiology-ceus/course/audiological-testing-w-severe-profound-40704
Audiological Testing with Adults with Severe to Profound Hearing Loss, in partnership with RIT/National Technical Institute for the Deaf
This course walks through a test battery for adults with severe to profound hearing loss, gathered from the collective experience of National Technical Institute for the Deaf (NTID) audiologists. Key differences from a standard test battery are highlighted.
auditory, textual, visual
129
USD
Subscription
Unlimited COURSE Access for $129/year
OnlineOnly
AudiologyOnline
www.audiologyonline.com
Audiological Testing with Adults with Severe to Profound Hearing Loss, in partnership with RIT/National Technical Institute for the Deaf
This course walks through a test battery for adults with severe to profound hearing loss, gathered from the collective experience of National Technical Institute for the Deaf (NTID) audiologists. Key differences from a standard test battery are highlighted.
40704
Online
PT60M
Audiological Testing with Adults with Severe to Profound Hearing Loss, in partnership with RIT/National Technical Institute for the Deaf
Presented by Erin Pickett, AuD, CCC-A/SLP, Vanessa Murphy, AuD, CCC-A
Course: #40704Level: Intermediate1 Hour
AAA/0.1 Intermediate; ACAud inc HAASA/1.0; AHIP/1.0; ASHA/0.1 Intermediate, Professional; BAA/1.0; CAA/1.0; Calif. SLPAB/1.0; IACET/0.1; IHS/1.0; Kansas, LTS-S0035/1.0; NZAS/1.0; SAC/1.0; TX TDLR, #142/1.0 Non-manufacturer
This course walks through a test battery for adults with severe to profound hearing loss, gathered from the collective experience of National Technical Institute for the Deaf (NTID) audiologists. Key differences from a standard test battery are highlighted.
3
https://www.audiologyonline.com/audiology-ceus/course/using-gsi-for-cochlear-implant-39682
Using GSI for Cochlear Implant Evaluations
This course is designed to educate audiologists on the practical workflow for patients who require cochlear implants. From Food and Drug Administration (FDA) approved indications and Medicare requirements to pre-op and post-op evaluations, audiologists will gain a clear understanding of the cochlear implant process.
auditory, textual, visual
129
USD
Subscription
Unlimited COURSE Access for $129/year
OnlineOnly
AudiologyOnline
www.audiologyonline.com
Using GSI for Cochlear Implant Evaluations
This course is designed to educate audiologists on the practical workflow for patients who require cochlear implants. From Food and Drug Administration (FDA) approved indications and Medicare requirements to pre-op and post-op evaluations, audiologists will gain a clear understanding of the cochlear implant process.
39682
Online
PT60M
Using GSI for Cochlear Implant Evaluations
Presented by Joseph Dansie, AuD
Course: #39682Level: Introductory1 Hour
AAA/0.1 Introductory; ACAud inc HAASA/1.0; AHIP/1.0; BAA/1.0; CAA/1.0; Calif. SLPAB/1.0; IACET/0.1; IHS/1.0; Kansas, LTS-S0035/1.0; NZAS/1.0; SAC/1.0
This course is designed to educate audiologists on the practical workflow for patients who require cochlear implants. From Food and Drug Administration (FDA) approved indications and Medicare requirements to pre-op and post-op evaluations, audiologists will gain a clear understanding of the cochlear implant process.
4
https://www.audiologyonline.com/audiology-ceus/course/empowerment-and-behavioral-insights-in-37124
Empowerment and Behavioral Insights in Client Decision Making, presented in partnership with NAL
Behavioral Insights can be used to understand and support hearing health decision-making, particularly in the appointment setting. An overview of empowering the individual's first awareness of hearing loss to hearing aid fitting and then to becoming an active hearing aid user will be covered.
auditory, textual, visual
129
USD
Subscription
Unlimited COURSE Access for $129/year
OnlineOnly
AudiologyOnline
www.audiologyonline.com
Empowerment and Behavioral Insights in Client Decision Making, presented in partnership with NAL
Behavioral Insights can be used to understand and support hearing health decision-making, particularly in the appointment setting. An overview of empowering the individual's first awareness of hearing loss to hearing aid fitting and then to becoming an active hearing aid user will be covered.
37124
Online
PT60M
Empowerment and Behavioral Insights in Client Decision Making, presented in partnership with NAL
Presented by Simon Alperstein, MSc BE, Paola Incerti, MAudiology, AAudA
Course: #37124Level: Intermediate1 Hour
AAA/0.1 Intermediate; ACAud inc HAASA/1.0; AHIP/1.0; BAA/1.0; CAA/1.0; Calif. SLPAB/1.0; IACET/0.1; IHS/1.0; Kansas, LTS-S0035/1.0; NZAS/1.0; SAC/1.0
Behavioral Insights can be used to understand and support hearing health decision-making, particularly in the appointment setting. An overview of empowering the individual's first awareness of hearing loss to hearing aid fitting and then to becoming an active hearing aid user will be covered.
5
https://www.audiologyonline.com/audiology-ceus/course/beyond-basics-interpreting-wideband-tympanometry-40708
Beyond the Basics: Interpreting Wideband Tympanometry with Confidence
Wideband tympanometry is a powerful tool—if you know how to use it. Join this session to learn practical interpretation techniques and gain the confidence to integrate WBT into your daily practice.
auditory, textual, visual
129
USD
Subscription
Unlimited COURSE Access for $129/year
OnlineOnly
AudiologyOnline
www.audiologyonline.com
Beyond the Basics: Interpreting Wideband Tympanometry with Confidence
Wideband tympanometry is a powerful tool—if you know how to use it. Join this session to learn practical interpretation techniques and gain the confidence to integrate WBT into your daily practice.
40708
Online
PT60M
Beyond the Basics: Interpreting Wideband Tympanometry with Confidence
Presented by Jackie Davie, PhD
Course: #40708Level: Intermediate1 Hour
AAA/0.1 Intermediate; ACAud inc HAASA/1.0; AHIP/1.0; BAA/1.0; CAA/1.0; Calif. SLPAB/1.0; IACET/0.1; IHS/1.0; Kansas, LTS-S0035/1.0; NZAS/1.0; SAC/1.0
Wideband tympanometry is a powerful tool—if you know how to use it. Join this session to learn practical interpretation techniques and gain the confidence to integrate WBT into your daily practice.


